AN APPEAL FOR SUPPORT
- We seek your support to meet expenses relating to some new and essential software, formatting of articles and books, maintaining and running the journal through hosting, correrspondences, etc. You can use the PAYPAL link given above. Please click on the PAYPAL logo, and it will take you to the PAYPAL website. Please use the e-mail address thirumalai@mn.rr.com to make your contributions using PAYPAL.
Also please use the AMAZON link to buy your books. Even the smallest contribution will go a long way in supporting this journal. Thank you. Thirumalai, Editor.
BOOKS FOR YOU TO READ AND DOWNLOAD
- THE ROLE OF VISION IN LANGUAGE LEARNING
- in Children with Moderate to Severe Disabilities - SANSKRIT TO ENGLISH TRANSLATOR ...
S. Aparna, M.Sc. - A LINGUISTIC STUDY OF ENGLISH LANGUAGE CURRICULUM AT THE SECONDARY LEVEL IN BANGLADESH - A COMMUNICATIVE APPROACH TO CURRICULUM DEVELOPMENT by
Kamrul Hasan, Ph.D. - COMMUNICATION VIA EYE AND FACE in Indian Contexts by
M. S. Thirumalai, Ph.D. - COMMUNICATION
VIA GESTURE: A STUDY OF INDIAN CONTEXTS by M. S. Thirumalai, Ph.D. - CIEFL Occasional
Papers in Linguistics,
Vol. 1 - Language, Thought
and Disorder - Some Classic Positions by
M. S. Thirumalai, Ph.D. - English in India:
Loyalty and Attitudes
by Annika Hohenthal - Language In Science
by M. S. Thirumalai, Ph.D. - Vocabulary Education
by B. Mallikarjun, Ph.D. - A CONTRASTIVE ANALYSIS OF HINDI
AND MALAYALAM
by V. Geethakumary, Ph.D. - LANGUAGE OF ADVERTISEMENTS
IN TAMIL
by Sandhya Nayak, Ph.D. - An Introduction to TESOL:
Methods of Teaching English
to Speakers of Other Languages
by M. S. Thirumalai, Ph.D. - Transformation of
Natural Language
into Indexing Language:
Kannada - A Case Study
by B. A. Sharada, Ph.D. - How to Learn
Another Language?
by M.S.Thirumalai, Ph.D. - Verbal Communication
with CP Children
by Shyamala Chengappa, Ph.D.
and M.S.Thirumalai, Ph.D. - Bringing Order
to Linguistic Diversity
- Language Planning in
the British Raj by
Ranjit Singh Rangila,
M. S. Thirumalai,
and B. Mallikarjun
REFERENCE MATERIAL
- UNIVERSAL DECLARATION OF LINGUISTIC RIGHTS
- Lord Macaulay and
His Minute on
Indian Education - In Defense of
Indian Vernaculars
Against
Lord Macaulay's Minute
By A Contemporary of
Lord Macaulay - Languages of India,
Census of India 1991 - The Constitution of India:
Provisions Relating to
Languages - The Official
Languages Act, 1963
(As Amended 1967) - Mother Tongues of India,
According to
1961 Census of India
BACK ISSUES
- FROM MARCH 2001
- FROM JANUARY 2002
- INDEX OF ARTICLES
FROM MARCH, 2001
- MARCH 2005 - INDEX OF AUTHORS
AND THEIR ARTICLES
FROM MARCH, 2001
- MARCH 2005
- E-mail your articles and book-length reports (preferably in Microsoft Word) to thirumalai@bethfel.org.
- Contributors from South Asia may send their articles to
B. Mallikarjun,
Central Institute of Indian Languages,
Manasagangotri,
Mysore 570006, India or e-mail to mallikarjun@ciil.stpmy.soft.net - Your articles and booklength reports should be written following the MLA, LSA, or IJDL Stylesheet.
- The Editorial Board has the right to accept, reject, or suggest modifications to the articles submitted for publication, and to make suitable stylistic adjustments. High quality, academic integrity, ethics and morals are expected from the authors and discussants.
Copyright © 2004
M. S. Thirumalai
THE ROLE OF VISION IN LANGUAGE LEARNING
in Children with Moderate to Severe Disabilities
Martha Louise Low, Ph.D.
The Role of Vision in Language Learning: Relationships between Visual Acuity, Looking Behavior, and Fast-Mapping of Novel Words onto Novel Objects in Children with Moderate to Severe Disabilities. A Ph.D. dissertation, University of Minnesota.
CLICK HERE FOR PRINTER-FRIENDLY VERSION.
CONTENTS
| A NOTE | CERTIFICATE |
| ACKNOWLEDGEMENTS | DEDICATION |
| ABSTRACT | LIST OF TABLES |
| LIST OF FIGURES | |
| CHAPTER 1 | INTRODUCTION |
| CHAPTER 2 | REVIEW OF THE LITERATURE |
| CHAPTER 3 | METHODS |
| CHAPTER 4 | RESULTS |
| CHAPTER 5 | DISCUSSION |
| REFERENCES | |
| APPENDIX A |
|
| APPENDIX B |
|
| APPENDIX C |
|
| APPENDIX D |
|
| APPENDIX E |
|
| APPENDIX F |
CLICK HERE FOR PRINTER-FRIENDLY VERSION.
*** *** ***
A NOTE
At the time that this dissertation was completed in 1999, a few observations seemed worthy of further study, and have implications for effective language intervention for children with disabilities.
The first, a study related observation found on pages 59-62 (see Results, section on Differences in Inaccurate Following by Ocular Coordination Groups), is the finding that children with and without disabilities who have binocular coordination problems (e.g., "cross-eyed" or "wall-eyed") did not match the researchers visual target ("looking behavior" in the study) and did not fast map novel words to novel objects. Informally, these children seemed to tolerate greater amounts of "labelling ambiguity," without requests for clarification. At the time of this study, binocular coordination had not been studied or assessed in relation to children with language learning problems.
The second, an observation unrelated to the study, has to do with participants with autism. Although these children often had perfect visual acuity (no refractive errors), they usually did not make good use of novel visual intormation (e.g., a new toy). Instead, it seemed that children with autism were much more receptive to tactile information (e.g., touching objects). Sometimes the same object was ignored (or treated with irritation) when presented visually. This is significant because many interventions for children with autism use visual stimuli. Although these are often successful interventions, tactile methods may be useful for earlier stages of language learning or for children who are severely affected. At the time of this study, few interventions used tactile stimuli, such as three dimentional objects for language intervention.
Additionally, it was observed that children with autism were difficult to direct using temporal visual cues (e.g., a "vanishing" symbol such as a gesture). The study noted on page 74 (see Results, section on Intervention Conditions, Graph of Mean Looking Scores for Eye Gaze, Head Turn, and Show Gesture) that children with autism often did not respond to "slight cues" such as shift in researcher eye gaze, or head turn. However, when a dramatic arm gesture (show gesture) was used, which "cut a larger arc" in the child's visual field, then the child responded. This also has implications for teacher-pupil interactions during language interventions.
CERTIFICATE
UNIVERSITY OF MINNESOTA
This is to certify that I have examined this copy of a doctoral thesis by
Martha Louise Low
And have found that it is complete and satisfactory in all respects, and that any and all revisions required by the final examining committee have been made.
__________________________
Name of Faculty Adviser
_____________________________
Signature of Faculty Adviser
_____________________________
Date
GRADUATE SCHOOL
The Role of Vision in Language Learning:
Relationships between Visual Acuity, Looking Behavior,
and Fast-Mapping of Novel Words onto Novel Objects
in Children with Moderate to Severe Disabilities
A THESIS
SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL
OF THE UNIVERSITY OF MINNESOTA
BY
Martha Louise Low
IN PARTIAL FULFILLMENT OF THE REQUIREMENTS
FOR THE DEGREE OF
DOCTOR OF PHILOSOPHY
Susan Hupp, Adviser
August, 1999
ACKNOWLEDGEMENTS
I would like to gratefully acknowledge the many people and agencies that made this project possible. Those who facilitated my endeavor were with me at every step in the process of this research project. The first stage involved those on my committee, who helped me to conceive and refine the project. Special mention belongs to Dr. Hupp, my adviser, for the endless stream of advice she was asked to produce on my behalf. I appreciated her keen ability to direct this work in an efficient manner. Also, my committee members, who graciously gave me time for presentations and consultations, included Dr. Bart, Dr. Bauer, Dr. Rynders, Dr. Samuels, Dr. Weiss, Dr. Wilderson, and Dr. Windsor.
Funds for the completion of this project were granted by the Graduate School Fellowship Office at the University of Minnesota in the form of a Doctoral Dissertation Special Grant. Their welcome support of this project has enabled the timely completion of this project. Funds were used for translation costs, transportation, materials, copies, and postage.
Dr. Knowlton facilitated the acquisition of necessary equipment and materials for the project (photorefraction camera and film). She also provided technical support for the vision screening photograph interpretation and woodshop expertise for the novel toys. Special Education Programs provided the use of a camcorder to videotape the children in this study. Other welcome donations of materials and services were made by My Yen Vo (Vietnamese translation), Louisa Watson (Spanish proofreading), Chin Ching-Yu (1/2 inch videotapes), Shelly Kothe (familiar children's toys), the Low's (use of the family car), and the public school districts (copies of consent forms). Many thanks to Sandi Cassavant who coded the data for Interrater Observer Reliability, and for her friendship throughout the graduate school experience.
The participants were recruited from seven public school districts and a church children's program: Minneapolis Public Schools, St. Paul Public Schools, Roseville Public Schools, Richfield Public Schools, Bloomington Public Schools, Independent School District #196, Independent School District #191, and Bloomington Assemblies of God Church. There were countless administrators and teachers at these locations who helped me with the research approval process, and the identification of potential participants. A huge thank-you to every parent or guardian who signed and returned a consent form for his/her child's participation in the study.
Last, but not least, I gratefully acknowledge my loving husband, Kevin Low, and my son, Derek Low for laboring with me, and for gracing my life with their presence, love, and encouragement.
DEDICATION
This work is dedicated to Jesus Christ of the Scriptures whom I thank so much for everything.
ABSTRACT
The purpose of this study is to examine a particular strategy of language learning in young children with moderate to severe disabilities. At around 18 months, typically developing children begin to use the direction of a speaker's eyes to guess the meaning of some words (e.g., objects). The use of this strategy coincides with increased vocabulary growth, and may be a result of strategy use. Children with moderate to severe disabilities may also use this strategy, but it is not known if problems with visual acuity (e.g., near-sighted, far-sighted), which are more prevalent in moderate to severe populations, may hinder their ability to reference a speaker in this way. Although language delays are known to be related to developmental delays and/or cognitive deficits, it is not known whether these delays or deficits indirectly affect language development through inefficient interpretation of degraded visual information (e.g., poor visual acuity).
This study videotaped children with and without disabilities as they participated in a novel word learning exercise. After verbalizations containing the novel word, participants' responses were coded for eye gaze targets (speaker and direction of eye gaze). In one condition, the researcher's gaze was directed toward the child's novel toy. In the other, the researcher's gaze was directed toward her own novel toy. After this, the child was asked to identify which of two objects was the novel toy. Additional intervention conditions were administered to children that were not able to accurately map the novel word to the novel object. These conditions accompanied the novel word with shifting eye gaze, head turns, and gestures. In addition, visual acuity was measured by the use of a photo-refraction camera. Photographs from this type of camera are able to reveal the presence and degree of many mild and severe visual conditions (e.g., near-sighted, far-sighted, misalignments [strabismus], amblyopia, cataracts).
Data were examined for associations between subject characteristics and (1) visual acuity, (2) visual targets, and (3) language mapping ability. Other analyses were made for associations between (4) visual acuity and visual targets, (5) visual targets and language mapping, and (6) visual acuity and language mapping.
LIST OF TABLES
Table 1 - Pearson correlations of the progressed age measures.
Table 2 - Spearman rank correlations of age progressions and looking variables.
Table 3 - Incidence of problems with acuity in the cognitive groups.
Table 4 - Incidence of ocular coodination problems in the cognitive groups.
Table 5 - Incidence of visual acuity problems for the diagnosis groups.
Table 6 - Incidence of ocular coordination problems for the diagnosis groups.
Table 7 - Spearman rank correlations of cognitive indexes and refractive error.
Table 8 - Frequencies of inaccurate mapping for cognitive groups.
LIST OF FIGURES
Figure 1 - Graph of the chronological ages of participants
Figure 2 - Graph of the cognitive age progressions of participants.
Figure 3 - Graph of show gesture find-it scores for the diagnosis groups.
Figure 4 - Graph of total find-it scores for the diagnosis groups.
Figure 5 - Graph of looking variables for the entire study sample.
Figure 6 - Graph of total sequential scores for cognitive group.
Figure 7 - Graph of total sequential looking for diagnostic groups..
Figure 8 - Scatterplot of refractive errors and cognitive indexes.
Figure 9 - Graph of inaccurate follows for occular coordination groups.
Figure 10 - Graph of inaccurate mapping for ocular coordination groups.
CHAPTER 1
INTRODUCTION
Children with moderate to severe disabilities are expected to have problems with language learning. Delayed language also is expected in cases of mental retardation (e.g., Down syndrome), with abnormal language development documented in cases of autism. Language research has given strong validation to social-interactionist theories of language development. In these paradigms, vision is an assumed, and necessary, skill in language development. The role of vision has been documented in various ways. For example, child vocabulary size has been correlated with mother-child joint attention characteristics (Goldfield, 1985-86-, Tomasello & Todd, 1983) and child joint attention skill in general (Morales, Mundy, & Rojas, 1998; Mundy & Gomes, 1998). Visual regard of a communication partner is part of the defining features of infant communication (Bates, Benigni, Bretherton, Camaioni, & Volterra, 1979). Effective language interventions usually emphasize joint attention activities or visual strategies for language learning (Matson, Benavidez, Compton, Paclawskyj, & Baglio, 1996).
There are a number of broad considerations to make when proposing a relationship between visual and language abilities. Children with refractive errors and normal cognitive abilities have no known difficulty with language learning. Children who are blind and have normal cognitive abilities are somewhat delayed in the development of speech but, eventually "catch up" to their sighted peers (Ferrell & Raver, 1991 - Hatton, Bailey, Burchinal, & Ferrell, 1997). However, children who have visual impairments and have cognitive disabilities are even more delayed in their language development. The cognitive deficits of this latter group of children may hinder them from making efficient use of their remaining vision. The concern of this research study is whether or not children with cognitive disability also are hindered in their language learning by the presence of refractive error (e.g., nearsighted or farsighted). Cognitive disabilities may hinder the efficient use of relatively good vision when refractive errors are present.
With regard to language learning, children with moderate to severe disabilities may experience cognitive overload in a number of ways. (1) Language is a complex, integrated process which usually involves many coordinated systems in the acts of comprehension and production (e.g., gesture, speech, and eye gaze) (Capirci et al., 1996; Caselli, 1983 -, Franco & Butterworth, 1996; Iverson et al., 1994; Zinober & Martlew, 1985). (2) Visual abilities are the most efficient means of determining the meaning of referents (e.g., "moon" or "yellow"). Tactile exploration of referents, for example, takes more time to process than visual exploration. (3) Refractive errors such as hyperopia and myopia may possibly add to the cognitive burden of interpreting the meaning of words. Refractive error could obscure certain important aspects of referents or social cues (e.g., direction of eye gaze). Refractive errors, particularly hyperopia, are much more common in children with moderate to severe disabilities than in normal populations.
A number of researchers have noted that the rate and quality of word learning at 12 months and 18 months is vastly different, even though children of both ages are similarly motivated to communicate (Nelson, 1973, as cited in Baron-Cohen, Baldwin, & Crowson, 1997; Baron-Cohen et al., 1997; Bakeman & Adamson, 1984). Children learn to speak at around 12 months of age, and combine two words at about 18 months of age. Word combinations herald a vocabulary spurt, where word learning occurs at a much faster rate. Differences in joint attention skill have been hypothesized to account for these differences in verbal production. At younger ages, children may use passive acquisition strategies or association with perceptual characteristics (e.g., visual appearance, functional use, association of a heard word and an event) (Baldwin, 1993; Baron-Cohen et al., 1997). During the middle of the second year, infants may switch to more active strategies of word learning (e.g., following speaker's direction of gaze to a novel referent).
A number of skill developments may aid in the language acquisition. Joint attention increases in complexity and length over the first couple years of childhood. At around nine months, infants engage in dyadic emotional play and participate passively in joint attention routines initiated by their mothers (Bakeman & Adamson, 1984). Triadic infant exchanges (mother, infant, and object/event) increase from an average of 7 seconds at 6 months of age to 34 seconds at 18 months of age.
Baldwin (1993) demonstrated that typically developing infants reference their communication partners in response to verbalization in similar ways throughout the second year. However, the infants did not use the information to map novel words to their referents. Only the 18-19 month old children were able to use the speaker's direction of gaze to successfully label novel objects in both conditions.
Baron-Cohen et al. (1997) used the same procedures to test word mapping strategies of children with mental retardation and children with autism (MA = 2 yrs.). Children with mental retardation performed almost as well as normally developing children, but children with autism exhibited poor use of speaker's direction of gaze strategy.
It could be that refractive errors hinder the use of visual information such as speaker's direction of gaze, because the eyes of the speaker are obscured (e.g., not in focus for the listener with refractive error). Furthermore, refractive error may obscure the referents being referred to by language. In either case, language learning could be further delayed, due to the lack of visual information. Cognitive disabilities may further compound this problem due to the inefficient use of visual information and the complex nature of language processing itself.
CHAPTER 2
REVIEW OF THE LITERATURE
This paper is an exploration of possible relationships between visual acuity and the acquisition of symbolic language. Children with moderate to severe disabilities are usually delayed in the acquisition of verbal or gestural language. Language interventions for these children focus on extension of social and cognitive abilities, but may inadvertently presume upon visual abilities in the process. Vision is often relied upon for comprehension, imitation, and production of diectic gestures. The meanings of words are also first decoded by visual determination of an environmental reference (e.g., fast mapping). Although the absence of vision (blindness) does not prohibit the development of language when cognitive abilities are normal, mild deficits in vision may present significant barriers to language learning in cases of developmental delay or mental retardation. When children have both cognitive deficits and significant visual impairments, progress toward all developmental outcomes is slower than that for visual impairments alone (Ferrell & Raver, 1991; Hatton, Bailey, Burchinal, & Ferrell, 1997). Would it be possible that refractive errors in children with cognitive delays could also present significant barriers to language learning? It seems logical to suggest that if referents and language partners are not clearly seen, this may unduly tax the limited cognitive abilities of children with developmental disabilities.
Language takes many gestural and verbal forms. This paper will be limited to those forms that are the most communicative and age appropriate for preschool children. Diectic gestures, symbolic gestures made with the hands, and words are used by normally developing children throughout their lives. Because communicative forms should be chosen with regard to current and future functioning, these forms are logical targets of interventions (Kaiser, 1993; Siegel-Causey & Wetherby, 1993) and will serve as the primary focus of this paper. Production of gestural and speech forms requires motor and/or hearing abilities, and may be beyond the capabilities of some children with moderate to severe disabilities. The alternative modes of communication taught in these cases will not be discussed here.
First, the normal development of presymbolic and symbolic skills will be traced and compared with specific examples of moderate to severe disabilities. The language skills of Down syndrome will serve as an example of cognitive impairments. Autism was chosen as an example of social and language impairment. The characteristics of these populations are representative of many preschoolers with moderate to severe disabilities. Early Childhood Special Education (ECSE) best practices favor social theories of language learning. This has practical significance for intervention, as the qualities of social settings are more easily manipulated than neural nets or linguistic acquisition devices. As such, "social comments" will be made on the process of language learning where applicable to the products of early language.
Next, the characteristics of visual refractive errors will be described. Normal development of visual abilities will be compared with selected moderate to severe disability populations. Many neurological and physiological attributes and abilities are needed in order to see. This paper will consider refractive errors (acuity), rather than color vision, functional vision (tracking, fixating, motor control of the eyes and head), or neurological impairments which may prevent visual processing. Of these, acuity is the most accessible to effective intervention. Three commonly occurring types of refractive errors will be considered: myopia, hyperopia, and amblyopia. Associations with intelligence and academic achievement will also be reviewed.
Finally, the role of vision in the language development process will be discussed. As mentioned before, even mild visual problems may create significant barriers to language acquisition when present with concurrent cognitive deficits. Current language intervention practices in ECSE will be addressed in terms of their sensitivity to refractive errors in populations with moderate to severe disabilities.
Normal Development of Verbal and Gestural Language
In all cultures the normally developing infant begins to speak single words at about 12 months of age, and to combine two words at about 18 months (Capirci, Iverson, Pizzuto, & Volterra, 1996; Slobin, 1972). Using verbal measures alone, this presents a rather abrupt picture of development. When incorporating information on the development of gestures, a much smoother picture emerges. This seems to be true of Western cultures, which are the settings for the research cited in this paper. In general, once forms are acquired, they are utilized throughout life, although the frequency of the various forms changes (Evans & Rubin, 1979; Pechmann & Deutsch, 1982). Reported below is research on production and comprehension of presymbolic and symbolic verbal and gestural development. Most studies agree on the criteria for verbal and deictic gestural forms. However, definitions for symbolic gestures vary widely (Haslett & Samter, 1997). An attempt has been made to use research that contributes specifically to symbolic gestures made with the hands, rather than facial expressions or whole body movements. However, because not much research exists in this area (Haslett & Samter, 1997), some studies reviewed here include differing measures. Comments on social theory are made which relate to the acquisition of presymbolic and symbolic products (e.g., use of the eyes for joint attention).
Definitions
The definition of a symbol is found in Bates, Benigni, Bretherton, Camaioni, and Volterra (1979): The comprehension or use, inside and outside of communicative situations, of a relationship between a sign and its referent, such that the sign is treated as belonging to and/or substitutable for its referent in a variety of contexts; at the same time the user is aware that the sign is separable from its referent, that is, not the same thing" (p.43). Words and some gestures qualify as symbols.
The definition of gestural forms is found in Erting and Volterra (1990, as cited in Capirci, Iverson, Pizzuto, & Volterra, 1996). Erting and Volterra have dichotomized gestures into two categories: 1) Diectic gestures (DG), which include gestures of giving, showing, requesting, and pointing. These gestures cannot be interpreted without inspection of the environment. 2) Representational gestures (RG), include hand movements, whole body movements, and facial expressions which represent an object. For instance, a child may use a motor action similar to that used with a familiar toy to represent that toy. RGs come to be associated with relatively stable meanings across different contexts of production. Because RGs represent the referent, even in their absence, they qualify as true symbols according to Bates et al. (1979). On the other hand, DGs "point to" a present referent in the environment and do not qualify as symbols.
Selected Features of Language Development
Diectic Gestures. Diectic Gestures appear at around 9 months of age when the infant begins to use presymbolic gestural forms for giving, showing, requesting, and pointing (Bates et al., 1979; Haslett & Samter, 1997). Communicative intent is usually defined by visual checking and persistence in the behavior until the presumed goal is met (Bates, 1979). Deictic forms arise from instrumental gestures which may not acknowledge their communicative effect upon another social agent. Instrumental behaviors are abbreviated, or shortened for production efficiency, and conventionalized into "agreed upon" forms for social efficiency. Requesting and giving appear to develop first, and later, showing and pointing (Bates, 1979; Zinober & Martlew, 1985).
Social awareness appears to motivate the production of diectic gestures (Sugarman, 1984). Diectic gestures coincide with the development of coordinated joint attention (Rogoff, 1990; Bakeman & Adamson, 1984) and are divided into two categories: (1) declarative gestures, which focus on sharing information, and (2) imperative gestures, which focus on requests. When the point is used for declarative purposes, young infants look at the communication partner immediately after pointing (Franco & Butterworth, 1996). By 14 months of age, the declarative point is preceded by looking at the communication partner and more frequent looking during the act of pointing. The point for requesting is not preceded by social reference of the communication partner.
More positive affect is associated with declarative joint attention behaviors than with requesting behaviors (Kasari, Sigman, Mundy, & Yirmiya, 1990; Mundy, Kasari, & Sigman, 1992). The pleasure that children have in communication may fuel the development of cognitive and social abilities involved in more precise forms of communication.
Declarative pointing is a precursor of the development of speech (Bates, 1979; Franco & Wishart, 1995; Haslett & Samter, 1997; Sugarman, 1983). Taken together, it could be hypothesized that declarative pointing is prerequisite or facilitative of speech development. However, some cultures of the world are not responsive to infants' prelinguistic communication and do not acknowledge the child as a communicative partner until after the child starts talking (Schieffelin & Ochs, 1983). The speech development of these children is not temporally different than children from Western cultures.
Words.Words appear at around 12 months of age, but the range of variation is quite wide (Bates, 1979; Thal & Bates, 1988; Bates et al., 1994). Words are first bound to specific contexts, and later become decontextualized (Rescorla, 1980). The majority of the first 50 words in Western studies are nouns and, secondly, verbs and then, social/personal words (e.g., "mommy," "hi") (Bates et al., 1994; Iverson, Capirci, & Caselli, 1994; Rescorla, 1981). Children with predominantly noun filled vocabularies (referential style) learn faster than those who have more personal-social words (expressive style) (Bates et al., 1994; Nelson, 1973, as cited in Haslett & Samter, 1997). Various researchers have tried to argue that words are learned or encoded by their perceptual characteristics (e.g., Clark) rather than their functional properties (e.g., Nelson), such as motor movements that are performed on objects (Haslett & Samter, 1997). However, in naturally occurring language learning contexts, these two are confounded. Few first words have solely one or the other property (Rescorla, 1980). Gopnik (1988; Haslett & Samter, 1997) infered from his study that "social" words appear first (e.g., "dada" and names of people), and then the "cognitive" words appear after that (e.g., "gone" and naming objects).
Deictic words (e.g., here, there, you, they, this, that) are late appearing and infants often use diectic gestures to substitute for or clarify diectic words (Clark & Sengul, 1978; Capirci et al., 1996). Several studies have found that diectic words were still less frequent than diectic gestures at 20 months (Capirci et al., 1996: Iverson et al., 1994). Clark and Sengul (1978) revealed a trend toward partial verbal mastery at 1 year to full mastery by 5 years. Diectic words have many verbal forms but, in the gestural mode, one form (point) can simply be used for many referents, thereby reducing cognitive requirements and improving social interactions.
The social environment may influence child output. The proportion of word types in children's vocabularies may be related to maternal input. Caregiver style that facilitated 12 month old child focus was associated with more nouns at 17 months (Tomasello & Todd, 1983). Directive styles were associated with more personal-social words. Goldfield (1985-86) claimed that it is the context that the dyad jointly constructs which determines vocabulary content: the referential style child is associated with interactions focused on objects, the expressive style child is associated with interactions focused on performance and participation. In a cross-cultural study, Tardif, Shatz, and Naigles (1997) demonstrated that caregivers of cultures whose speech contained more nouns than verbs had children who produced accordingly, and vice versa. However, Caselli et al. (1995) argued that some languages (e.g., Korean and Chinese) lack the complex morphology that might be expected to slow the acquisition of verbs. They claim that oriental children are learning verbs sooner, which makes them appear to have larger verb categories at the same age. In either case, both studies show that child language is related to characteristics of maternal cultural.
Symbolic gestures
Symbolic gestures, like words, also appear at around 12 months (Acredolo & Goodwyn, 1988). Like words, symbolic gestures also undergo a process of decontextualization (Acredolo & Goodwyn, 1988; Caselli, 1983). These gestures can be spontaneously produced from an existing motor repertoire (e.g., pantomime of sniffing a flower to represent "flower") (Acredolo & Goodwyn, 1988). Symbolic gestures seem to incorporate functional elements rather than perceptual elements (Acredolo & Goodwyn, 1988). Word classes corresponding to the meanings of first gestures are roughly equivalent to first words; nouns predominate, verbs are second (Iverson et al., 1994). Parent interviews indicated that the gestural symbols are acquired for words that are not yet a part of child verbal vocabulary and that, once words are acquired for those items or actions, the gestures are no longer used (Acredolo & Goodwyn, 1988). Iverson et al. (1994) reported that gestural and verbal vocabularies had only a 10% overlap at 16 months of age. The frequency of gesturing was found to be at its peak around the time that infants acquired 10 words, and 80% of gestures were made by the acquisition of 25 words (Acredolo & Goodwyn, 1988). At that point gesture production became greatly reduced, although it was present throughout the 50 word period (Capirci et al., 1996). Combined findings indicate that as words increase in number, gestures decrease (Acredolo & Goodwyn, 1988; Capirci et al., 1996; Iverson et al., 1994).
The frequency of gesturing can be expected to vary according to the culture. As stated before, some cultures emphasize verbal forms of communication (e.g., Kaluli) (Sugarman, 1983; Schieffelin & Ochs, 1983). Research in cultures that gesture more (e.g., Italian) have shown differences with cultures that gesture less (e.g., American). Children from Italy showed a greater prevalence of gestural communication than that found in the American samples (Capirci et al., 1996, commenting on Goldin-Meadow & Morford, 1985). Factors within cultures that produce variability in onset may be related to caregiver availability. Both gesture onset and speech onset have been shown to be correlated with the number of hours per week spent in day-care; the higher numbers of hours were associated with higher ages of onset in both modalities (Goodwyn & Acredolo, 1993). Maternal availability would also explain why first-born children gestured more in Acredolo and Goodwyn's study (1988). Some symbolic gestures may be learned from adult input or from caregiver routines (Acredolo & Goodwyn, 1988; Capirci et al., 1996; Haslett & Samter, 1997; Zinober & Martlew, 1985). In general, the data seem to indicate that infants' communication partners determine child input, which may also be predetermined to some degree by partners' culture.
Timing of words versus gestures
A commonly debated issue is whether or not verbal and gestural symbols share a common cognitive base. Studies are inconclusive as to whether symbolic gestures precede first words. If onset is simultaneous for both modalities, this indicates support for maturation of some cognitive mechanism, which would employ either modality in the task of symbolic representation (Goodwyn & Acredolo, 1993; McNeill, 1985). Proponents of a "sign advantage" claim that gestures are easier to employ in tasks of reference because motor schemes are well established by the time infants develop referential abilities (Piaget, 1962, as cited in Thal & Bates, 1988). Werner and Kaplan (1963, as cited in Capirci et al., 1994) also theorized that "distancing" occurs through initial use of a motor action to represent a referent. Greater "distancing" is then made possible through use of the verbal mode. Goodwyn and Acredolo (1993) reported that a small, but statistically significant, gestural advantage exists (about 3 weeks, range .6-3.6 months). Even without a proven "sign advantage" the replacement of gestural vocabulary with verbal vocabulary suggests that infants find some words too taxing to produce until further cognitive development has taken place (Acredolo & Goodwyn, 1988). Furthermore, gesture advantage may be related to maternal sensitivity, a variable which attempts to measure caregiver responsiveness to infant signals for aid or comfort. The infants in Goodwyn and Acredolo's (1993) study who demonstrated a gesture advantage of at least one month differed from those who did not demonstrate an advantage, based on higher maternal education, a variable noted for correlation with maternal sensitivity (Bakeman & Adamson, 1986; Seifer, Schiller, Sameroff, Resnick, & Riordan, 1996).
Two-word Combinations. At around 18 months the typically developing child combines two words in a single utterance, often after a "critical mass" of 50 words have been acquired (Bates et al., 1994; Capirici et al., 1996; Slobin, 1986). Deaf infants also combine two signs (referential symbols) at the same time that hearing infants combine two words (Caselli, 1983; Goodwyn & Acredolo, 1993). A few studies have attempted to document the way in which two word combinations are associated with symbolic gestures. Authors of these studies often emphasize the individual variation exhibited by their subjects when attempting to make generalizations from the data (Capirci et al., 1996; Iverson, et al., 1994; Zinober & Martlew, 1985).
The most salient finding is that gesture+word forms are used throughout the second year, before and after the acquisition of two-word combinations (Capirci et al., 1996; Goldin-Meadow & Morford, 1985; Iverson et al., 1994; Zinober & Martlew, 1985). Goldin-Meadow and Morford (1985) also indicated that gesture+gesture forms also appear before the two word stage, although much less commonly. Two diectic gestures (give + point) make up the majority of these forms. These researchers report that gesture+gesture forms disappear once word+word combinations appeared. Specific types of gesture+word combinations were measured by Capirci et al. (1996) and Zinober and Martlew (1985). Data suggest a developmental trend where combinations of the two modes are first redundant, and, later, supplementary in their message content. Supplementary gesture+word combinations may appear just prior to two-word combinations (Capirci et al., 1996).
Before the end of the first year, diectic gestures are a primary means of intentional communication (Bates, 1979). Words and gestures appear separately at first, and then become increasingly coordinated during the second year (Zinober & Martlew, 1985). At the beginning of the first year, diectic or symbolic gestures and words are used equally often for communication tasks and finally, gestures take a more supportive role to verbal communication (Iverson et al., 1994; Zinober & Martlew, 1985). Especially illustrative of this is that the number of symbolic gestures decreases as words replace them (Acredolo & Goodwyn, 1988; Iverson et al., 1994). The symbolic gestures that continue to be produced seem to fulfill more supportive roles in communication (e.g., adjectives for number or size) (Iverson et al., 1994).
Children may show a preference for gestural or verbal modality before two-word combinations develop, regardless of the number of vocabulary items in either mode. These preferences were demonstrated at 16 months in Capirci et al.'s (1996) study but, by 20 months, the verbal mode was strongly preferred over the gestural mode. Iverson et al. (1994) measured increases in diectic gestures (68-80%) and diectic words (5-13%) at the same ages (16 and 20 months). Pointing accounted for the majority of diectic gestures at both age points.
From prelinguistic communication to two word combinations, the communication of normally developing children fulfills socially oriented purposes of behavior regulation, social interaction, and joint attention (Wetherby, Cain, Yonclas, & Walker, 1988). In normal children, these functions seem to emerge simultaneously. Context also influences the functions of child productions and confounds comparisons of study measures (Wetherby & Rodriguez, 1992; Zinober & Martlew, 1985).
Vocabulary prediction
Attempts have been made to predict vocabulary size toward the end of the second year by earlier verbal measures taken during the beginning of the second year. Predictions are improved by adding gestural measures or verbal comprehension measures (Thal & Bates, 1988). Frequency of gesture+word combinations have been correlated with later vocabulary size (Capirci et al., 1996; Goodwyn & Acredolo, 1993). Both age at the acquisition of 10 words and the propensity to imitate gestures at 17 months were related to later verbal vocabulary size (Acredolo & Goodwyn, 1988). Ability of 1.6 yr. olds to follow the gaze and point of an experimenter is positively correlated with receptive language development (Mundy & Gomes, 1998). Receptive vocabulary at 17 months is correlated with the tendency of infants to follow a point and gaze of a tester at 14 months (Mundy & Gomes, 1998).
Comprehension
Differences exist between the sizes of verbal comprehension and production vocabularies: children understand more words than they can produce (Benedict, 1979; Rescorla, 1981). Two stages have been described in the vocabulary development of 1-2 yr olds (Goldin-Meadow, Seligman, & Gelman, 1976). First, the children said fewer nouns than they understood. They said no verbs, although they understood many. In the next phase, they said virtually all the nouns they understood plus their first verbs. Children in the one-word stage can comprehend and respond to two-word directions, even when they describe actions unfamiliar to the child (e.g., "kiss book") (Sachs & Truswell, 1978). Children at 18 months of age accept novel gestures or words as communication (Namy & Waxman, 1988). Older infants at 26 months of age could understand novel words, but not novel gestures, unless additional practice was given.
Studies have been conducted to assess whether or not children use extra-linguistic cues to improve comprehension. Although gestures improve verbal comprehension in adults, gestures don't seem to help children under two years of age in the same way. Maternal gesturing seems to increase child attention, but not understanding (Schnur & Shatz, 1984). Children in Morford and Goldin -Meadow's study (1992) performed better with familiar diectic gestures (e.g., "point" & "give"), rather than "shake" and "throw" gestures, which were new to them. Morford and Goldin-Meadow demonstrated that children's comprehension was improved by gestures, particularly the redundant type, where words and gestures carry the same meaning. Supplementary gestures also produced higher levels of performance than the word alone condition.
The inconsistency of findings on whether or not gestures improve comprehension of words may be a problem with the chosen measures. The studies above used acts of compliance to indicate comprehension in children under one year old. It has been demonstrated that children aged 1.4 - 1.6 were more likely to act if a gesture accompanied a directive or was used alone (Allen & Shatz , 1983). In this study, children tended to respond in the mode that the directive/question was given in. If a gesture accompanied speech, the child responded with some kind of action; if no gesture accompanied the request, words were more often used. The tendency to act when gestures are used may be instrumental in the long term task of word comprehension.
Other studies have attempted to reveal whether the child relies on one modality more than another in order to decipher messages (Allen, 1991; Allen & Schatz, 1983; Thompson & Massaro, 1986). When presented with conflicting verbal and gestural cues subjects tend to respond in the mode in which the directive was given (Allen & Shatz, 1983). Thompson and Massaro (1986) systematically varied the ambiguity of simultaneous gestural and verbal directives. Preschool age subjects (2.5 - 3.11 yrs.) were unable to use the clear source of information to decode messages. The ability to integrate sensory information improved with age.
IMPAIRED DEVELOPMENT OF VERBAL AND GESTURAL LANGUAGE
Children with mild disabilities and resulting language delays may not be identified until three years of age (Rice & Schuele, 1995). This puts children's development at a disadvantage, because much time is lost when interventions might have been taking place. Children with moderate to severe disabilities, on the other hand, are usually identified closer to birth (Morrison & Polloway, 1995). Language delays or impairments are often expected, in spite of the best efforts of prescribed intervention services. The notable exception is autism, a severe disability which typically has an onset before 36 months of age. Individuals with moderate to severe disabilities have a wide range of different disabling conditions. Each child shows a great deal of variation in his/her manner of communication (e.g., comprehension mode, production mode, contexts of participation). A comprehensive picture of symbolic communication development in the moderate to severe range of functioning is nearly impossible. Instead, specific conditions, such as Down syndrome and autism, are provided as examples of possible effects of moderate to severe disabilities on language development.
DOWN SYNDROME
Language development
Down syndrome is a genetic condition that causes varying degrees of mental retardation. Among children with Down syndrome, verbal and gestural language development is much slower and shows a great deal of individual variation (Franco & Wishart, 1995; Rondal, 1988). Although many cognitive and developmental skills are delayed, language is delayed even more so. Pointing and first words may appear at 20-24 months. Two word combinations could appear at 3-6 years. The two-word combinations of children with Down syndrome are similar to that of normal children at the same stage of language development (Rondal, 1988). Comprehension vocabulary contains more items than receptive language vocabulary. However, compared to receptive language matched peers, children with Down syndrome produce more gestures (Caselli et al., 1998; Mundy, Sigman, Kasari, & Yirmiya, 1988). This means that children with Down syndrome gesture more than expected for their level of language development. As Zinober and Martlew (1985) suggested, perhaps this is so because of the extended delay in verbal production. However, the frequency of nonverbal requests is less than would be expected. Nonverbal requests are correlated with later expressive language scores (Mundy, Kasari, Sigman, & Ruskin, 1995; Mundy et al., 1988).
Social aspects of language development
Children with Down syndrome demonstrated social awareness in the execution of their diectic gestures (Franco & Wishart, 1995). They exhibited the skills of normally developing children in declarative gestures, in that they looked at the partner before pointing. The subjects' mothers were the recipients of more communicative gestures than were unfamiliar peers. Unfamiliar peers, however, received more social referencing looks than did the mothers, which may indicate that the subjects were aware of the greater need to monitor their attention.
Children with Down syndrome do not differ significantly from normal subjects on measures of coordinated joint attention (Kasari, Freeman, Mundy, & Sigman, 1995). However, during joint attention episodes, they spend more time looking at a communicative partner's face than do nonretarded children (Kasari, Mundy, Yirmiya, & Sigman, 1990). Studies on maternal input have not revealed any differences between mothers of normal and impaired children on the proportion of nouns or diectic words (Cardoso-Martins & Mervis, 1990).
Language intervention
Interventions for children with Down syndrome have focused on prelinguistic requests and comments (Warren, Yoder, Gazdag, Kim, & Jones, 1993). Nursery rhymes and parent administered milieu techniques have been used to teach vocabulary (Glenn & Cunningham, 1984). Successful interventions have also used redundant word + gesture combinations (Reich, 1978) and two word combinations modeled during play (Jeffree, Wheldall, & Mittler, 1973). Salmon, Rowan, and Mitchell (1998) contrasted the more didactic milieu techniques with responsive (interactionist) techniques in an alternating treatments design. Milieu produced higher rates of intentional communication, and interactionist techniques yielded a more balanced distribution of comments and requests and discourse functions (e.g., initiations and responses).
AUTISM
Language development
Autism is a condition that causes disordered language and social development. This means that several developmental domains are not commensurate with a child's chronological or mental age, or that development proceeds at an erratic rate (P. Pulic, psychologist, personal communication, January 1998; S. Patterson, psychologist, personal communication, January 1998). Because of diagnostic changes made over the past 50 years, children who receive an autism label do so for a wide range of behaviors (APA, 1994; Cohen, Volkmar, & Paul, 1986). Onset of the disorder may occur after verbal language has begun to develop. They may exhibit echolalic (communicatively nonfunctional) speech or repetitive behaviors. Children with autism may or may not be mentally retarded. Those with lower IQs are likely to be mute, while those with higher IQs are likely to have some functional language (Waterhouse et al., 1996).
Deictic gestures of request are more frequent than those for declarative purposes (e.g., show, point) (Mundy, 1995; Mundy & Sigman, 1989; Wetherby & Prutting, 1984). If pointing does develop, it may be used for requesting, rather than declaring (Goodhart & Baron-Cohen, 1993; Franco, & Wishart, 1995; Mundy, Sigman, Ungerer, & Sherman, 1986). If a declarative pointing gesture develops, functional speech may appear soon afterwards, in accordance with the normal sequence of development (Sugarman, 1983).
Spontaneous use of symbolic gestures is inconsistently noted by researchers and interventionists. For instance, social gestures of greeting are less likely to be offered spontaneously, and less likely to be returned if given the opportunity (Hobson & Lee, 1998). Communication interventions often focus on teaching these gestures as an alternative to speech (see below).
If verbal skills do develop, they may do so in unconventional ways. Words or phrases that are produced do not appear to be functional communication. Whole chucks of language heard from a caregiver, such as a phrase or sentence, may be parroted over and over without regard to their uncommunicative effect (echolalia). It is often difficult to ascertain the idiosyncratic meaning of these utterances. Some repetitions are thought to occur when communication is not understood (Light, Roberts, Dimarco, & Greiner, 1998; S. Merzer, psychologist, personal communication, January 1998). Mitigation of the echolalic response has also been associated with the ability to learn speech (Bebko, 1990). However, some mute autistics have acquired language after long periods of time (Windsor, Doyle, & Siegel, 1994).
Comprehension of verbal or gestural language in children with autism has been used to develop screening measures for autism. Gestural joint attention (a point) could correctly identify 70-80% of the autistic and the language age matched kids at both the initial and follow-up assessments (Mundy, Sigman, & Kasari, 1990). Integration of sensory information may be a characteristic weakness of autism (Pierce, Glad, & Schreibman, 1997). A number of researchers have suggested that cognitive processing problems are minimized by using visual formats (Bebko, 1990). Interventions typically make use of visual presentation to support comprehension (Dijkxhoorn, Berckelaer-Onnes, & Ploeg, 1996; Light et al., 1998; Peterson, Bondy, Vincent, & Finnegan, 1995).
Social aspects of language development
Children with autism are noted for a lack of eye contact (Hobson & Lee, 1998; Willemsen-Swinkels, Buitelaar, Weijnen, & van Engeland, 1998), which is a precursor to normal communication development (Bates, 1979; Bakeman & Adamson, 1984; Sugarman, 1983). Preschool age autistic subjects returned fewer gazes than did developmentally delayed or normal subjects (Willemsen-Swinkels et al., 1998).
Autistic children's joint attention behaviors are worse than those of their developmentally delayed peers matched for language, chronological age, and mental age and language (Mundy, Sigman, Kasari, 1990; Roeyers, Van Oost, & Bothuyne, 1998). They do not display higher levels of affect during joint attention when compared with requesting episodes (Kasari, Sigman, Mundy, & Yirmiya, 1990; Mundy, Kasari, Sigman, 1992), contrary to normal development. In fact, autistic subjects were lacking in affect, regardless of context.
If children with autism do produce diectic gestures, the function of these gestures usually is to request, rather than declare/refer (Goodhart & Baron-Cohen, 1993; Mundy, 1995; Mundy, Sigman, Ungerer, & Sherman, 1986; Mundy, Sigman, & Kasari, 1994; Wetherby & Prutting, 1984). If declarative gestures are used, preschool autistic subjects look at the communication partner after making the gesture (Willemsen-Swinkels et al., 1998), contrary to Down syndrome and normal development. Just as in normal development, where language skills are associated with mental age, autistic deficits in the form and function of prelinguistic communication are also related to level of developmental attainment (Mundy, Sigman, & Kasari, 1994; Willemsen-Swinkels et al., 1998).
Verbal input of mothers to their autistic children follows child focus to the same degree as mothers of normal children (Watson, 1998). However, in a study by McArthur and Adamson (1996), unfamiliar adult partners gave fewer conventional bids for attention to autistic subjects than to language and age matched language delayed subjects. Instead, child attention was redirected using literal bids (e.g., interposing an object in line of gaze). The adults were experienced in language disorders, but did not know child diagnoses.
Joint attention behaviors have also been associated with predictive outcomes on language measures (Mundy, Sigman, Kasari, 1990; Willemsen-Swinkels et al., 1998). Words may also be incorrectly learned because the child is not able to follow the speaker's line of gaze, and uses his/her own line of sight to map word meanings (Baron-Cohen, Baldwin, & Crowson, 1997). The fact that children with autism experience social deficits that seem to hinder language acquisition, and that improvements in social functioning bring improvements in language functioning, is highly suggestive of the necessity of social contributions to language development (Rogers-Warren & Warren, 1984; Sugarman, 1983).
Language intervention
Interventions which involve a communication partner have attempted to stimulate normal development (e.g., facilitate speech or joint attention) or to teach the use of alternative modes of communication (e.g., sign or picture symbols). Although interventions may produce more functional language, deficits in social interactions usually persist throughout the life-span. Below are interventions for facilitating normal communication development.
Facilitating joint attention for children with autism is often difficult, due to autistic behaviors and limited attention spans. Most interventions imply facilitation of joint focus, but vary in the way in which this is accomplished. Behavioristic methods have been successful in teaching language skills (Lovaas, 1997; Harris & Weiss, 1998; Matson, Benavidez, Compton, Paclawskyj, & Baglio, 1996). These methods provide stable (even rigid) contexts where the focus of attention is interventionist supplied. Once speech is taught using behaviorist methods, it is often not generalized to other contexts outside of the intervention setting (Matson et al., 1996).
Another program that specifically targets joint focus uses child focus to build joint attention skills ("Floor Time," Greenspan & Wieder, 1998). Turn-taking skills and the affective side of interactions are given a strong emphasis in this program. Few research studies exist which support child responsive interventions (Hewill, 1998), but there is a growing number of case studies that report improvements in social or language skills (Greenspan & Wieder, 1998; Rollins, Wambacq, Dowell, Mathews, & Reese, 1998).
Milieu techniques are better supported by the research literature. Natural language teaching paradigms lie somewhere between the ultra-structured and ultra-unstructured approaches. Sessions emphasize child focus by utilizing an object that the child is interested in. The therapist plays with the object and verbally models the target language while waiting for the child to respond. Natural conversation is preserved, and child response is optional. The technique has demonstrated improvements over serial trial techniques (Koegel, O'Dell, & Koegel, 1987) and has been effective in reducing disruptive behavior during language intervention (Koegel, Koegel, & Surratt, 1992). Incidental teaching and time delay techniques also improved generalization (Matson et al., 1996).
Few intervention studies have addressed receptive language, a common deficit in children with autism (Matson et al., 1996). However, many interventions use visual means or object attainment to support comprehension. Multi-modal presentation of speech and sign has demonstrated gains over speech alone or sign alone methods (Matson et al., 1996). However, both sign and speech use transient symbolic mediums which employ a temporal sequence. This may present difficulties for autistic children's attention deficits. Peterson et al. (1995) demonstrated the superiority of using a static visual system (pictures) for interventions with autistic children. The picture only conditions had greater gains than speech alone or a combination of visual and speech, and had lower rates of disruptive. Perhaps the positive results of the picture only condition are due to the support they lend to joint attention deficits. Autistic children's difficulties in comprehension and/or lack of integration of sensory information may also be due to joint attention deficits (Pierce, Glad, & Schreibman, 1997). There has been a long-term established emphasis on visual strategies for supporting communication intervention for children with autism (Bebko, 1990; Dijkxhoorn, Berckelaer-Onnes, & Ploeg, 1996; Hodgeson, 1995; Light et al., 1998; Peterson, Bondy, Vincent, & Finnegan, 1995).
Refractive Errors in the Normal Population
Although language development is normally complete by the age of five years (Haslett & Samter, 1997), this is not so for children with moderate to severe disabilities. Therefore, the refractive development of the eye will be reviewed from birth to maturity, in order to provide information on possible visual states that may exist for children who are delayed in their language development. Research demonstrates that refractive errors change over the life span of an individual. However, these studies are complicated by the various methods of assessing refractive errors. Three common types of refractive errors will be presented: (1) Myopia, (2) Hyperopia, and (3) Amblyopia. Astigmatism is not addressed because it often co-occurs with other refractive disorders. Correlations of refractive errors with IQ and achievement scores are discussed along with theories that explain these associations. Interventions for mild vision problems are briefly outlined.
ASSESSMENT
Screening methods for vision problems include the familiar Snellen Chart (e.g., E-chart). The procedure used for this quick assessment and its variants requires that the chart be placed at a distance of 20 feet from the assessed. This procedure will certainly screen for near-sighted (myopic) people, but far-sighted (hyperopic) people will generally pass this test (Getman, 1985; Kirschen, 1954; Manley & Schuldt, 1970). Myopia causes the visual image to be focused in front of the retina, causing difficulty discerning a given target (Skrtic, 1995). This effect is ameliorated by viewing targets at closer distances. Hyperopia causes the visual image to be focused in back of the retina, also causing difficulty discerning the image. This effect is ameliorated by viewing targets at greater distances. Testing of each eye independently can sometimes reveal differences in acuity. Also, eye chart assessments do not reveal any information about how a subject sees at close distances, which is where most school work is done.
A more thorough assessment is done by the optometrist or ophthamologist. These professionals define refractive error with a unit of measure called a diopter (D). Plus numbers (+) indicate hyperopia, while minus (-) numbers indicate myopia. Zero (0) refractive error indicates normal vision. It takes a small measurement to define myopia, usually -.5 D. There is less agreement about defining hyperopia, which ranges from +1.25 D to +3.00 D. Because of the lack of agreement, two researchers could conceivably report different percentages of myopic, emmetropic (normal), and hyperopic participants when reviewing the data from a single sample (Hirsch, 1964).
During assessment the eyes (actually the irises) are usually dilated with a drug. This is referred to as the cycloplegic method. Cycloplegia has been criticized because it can overestimate refractive errors. Non-cyclopeged methods involve relaxing the irises by other means, such as placing the assessed in a dark room. (Proponents of cycloplegia say that "dark refraction methods" under-estimate the amounts of refractive error.) There is generally one (1) diopter (D) of difference between cyclopleged and non-cyclopleged techniques (Howland, 1988). However, in infant populations these methods are not well correlated (Maino & Gerhard, 1984, cited in Kohl & Samek, 1988).
In addition to these disparities of measurement methods, the boundaries that determine the differences between hyperopia, emmetropia (normal vision), and myopia are placed inconsistently by various researchers.
SELECTED FEATURES OF VISUAL DEVELOPMENT
Myopia, Emmetropia, and Hyperopia
1. Birth: The erratic gaze and blinking reflex of newborns make the assessment of their vision difficult (Howland, 1991). Howland's (1991) summary of studies on refractive errors in infants report that infants are generally myopic (near-sighted) when using dark refraction procedures and hyperopic if cycloplegic methods are used. In addition to the unreliable assessment of infants, different genetic endowment may dictate the varied responses to cycloplegia. Newborns also lack the focusing power of the irises, which is called accommodation (Getman, 1985; Howland, 1988). Accommodation is a function of the autonomic nervous system (Howland, 1991; Getman, 1985), and quickly develops to adult levels by six months of life.
2. First Year: Refractive assessments are more reliable as infants age, because gains in head control facilitate refractive techniques (Howland, 1991). The distribution of refractive errors becomes more restricted toward the close of the first year. This may be because of accommodation gains, and/or it may be because of the refractive qualities of the lens, cornea, and axial length of the eye (Howland, 1991).
3. First Five Years: Cyclopleged findings indicate that mean refractive errors tend toward hyperopia over the first five years (Howland, 1991). As children age, they become increasingly hyperopic, and this trend decelerates as they approach five years of age. Noncyclopleged results show no increasing trend toward hyperopia, but do indicate that children are slightly hyperopic (+.75 D). Other cyclopleged results have shown a mean of +1.27 D in children 1, 2, and 3 years old (Dobson, Fulton, Manning, Salem, & Petersen, 1981), and low hyperopia for 0-2 year olds (Kohl & Samek, 1988). The distribution of refractive errors continues to narrow in range over the first five years (Howland, 1991).
4. 5 Years-Adolescence: Most children enter kindergarten with some measurable amount of hyperopia (Hirsch, 1964; Howland, 1991). As children approach puberty, the eyes begin a trend toward myopia (Hirsch, 1964). Children who enter kindergarten with +.5 D to +1.25 D of hyperopia are likely to be emmetropic at adolescence. Children who are emmetropic during kindergarten tend toward myopia as they become adolescents. Children who enter kindergarten with measurable amounts of myopia tend to become more myopic as they age. Those with greater amounts (+3.0 D) of hyperopia will tend to remain hyperopic. (Those that are likely already myopic tend to remain so.) Twelve percent (12%) of students entering grammar school wear corrective lenses, 28% of those students wear corrective lenses upon graduation (Hirsch, 1951-52, as cited in Kolb, 1962).
Amblyopia
Amblyopia is a condition where both eyes do not focus in the same way (Ameder, Peck, & Howland, 1990). Incidence varies greatly depending on the study sample. Estimates range from 1-5% of the school age population (Ameder, Peck, Howland, 1990; Ottar, Scott & Holgado, 1995). There are two subtypes of amblyopia. (1) Anisometropia amblyopia, refers to the inability of the two eyes to accommodate equally on a given target (usually one eye is out-of-focus). (2) Strabismus, refers to a lack of binocular alignment or coordination. One eye may be permanently or intermittently turned inward (esotropia), outward (exotropia), upward (hypertropia), or downward (hypotropia). Amblyopic conditions may also cause the cognitive suppression of visual information from the eye that performs badly. Additionally, gross and fine motor problems are often associated with amblyopia (Slavik, 1982).
Some theories propose that amblyopia begins with a lack of accomodation during early development (Ameder et al., 1990). This can result in a lack of neurological "feedback loops," which are necessary for the reinforcement of binocular focus and alignment. Animal studies have shown that errors in accommodation can cause both kinds of amblyopia (Ameder et al., 1990; Howland, 1988). These errors are usually induced by prolonged covering of one eye. However, this progression has only been inferred inconsistently in human populations (Ameder et al., 1990; Ottar, Scott & Holgado, 1995). It may be just as likely that strabismus causes anesometropia/amblyopia (Ameder et al., 1990; Howland, 1991). Amblyopia is usually detected by the age of five years (Ameder et al., 1990).
TREATMENTS
Prescriptive treatments
Myopic people frequently are given prescriptions for refractive errors as small as -.5 D. For hyperopia, glasses are rarely prescribed, unless the errors are at least +2.0 or +3.0 D (M. Knowlton, Ph.D., class on vision screening, University of Minnesota, October 1998). This is especially true of children, whose eyes are still changing in their refractive characteristics. Because of the adverse effects of hyperopia on reading, Rosner and Rosner (1997) recommended prescription of glasses for +1.25 D or more of hyperopia (see below for a more complete discussion of refractive error and reading scores). The prescription of glasses for anesometropia is regarded by some as a possible prevention of amblyopia (Almeder et al., 1990). Some clinicians discourage the use of corrective lenses for strabismus because eye alignment problems may become correspondingly worse (Carter, 1964).
Amblyopia is often treated by "occlusion," which prescribes covering the better eye, in order to strengthen the worse eye (Mohindra, Jacobson, Zwaan, & Held, 1983). Occlusion is effective in increasing the acuity of the worse eye, but the occluded eye temporarily decreases in acuity. Monoccular occlusion also adversely affects alignment (Herman, Tauber, & Roffwarg, 1974). Strabismus is also effectively treated with surgery (Norcia, Hamer, Jampolsky, & Orel-Bixler, 1995).
Behavioral treatments
Vision Therapy, a highly controversial treatment, has been used to address problems with myopia (Blount, Baer, & Collins, 1984), hyperopia (Leung, Yap, & Lagrow, 1992), amblyopia (Flax, 1993). Getman (1985) explained that eye exercises have been known to help, but former explanations were implausible. Eye exercises do not increase muscle strength, because the eyes are already 100 times stronger than they need to be. Also, because accomodation is controlled by the autonomic nervous system, it is not subject to fatigue. Getman (1985) suggested that changes in acuity have taken place because the mind has been trained to perceive visual targets differently. The exercises taught the subject to use available visual information more efficiently. Behavioral techniques for acuity do not generalize well. In addition to training exercises, vision therapists sometimes prescribe lenses that over or under correct refractive errors in an attempt to bring long-term correction to vision problems.
COROLLARY FINDINGS
Intelligence scores
Students with myopia have been associated with higher intelligence test scores than students with normal or hyperopic vision, even after controlling for age, gender, father's occupation, and/or nonverbal performance IQ (Hirsch & Nadell, 1958; Manley, & Schuldt, 1970; Williams, Sanderson, & Share, 1988). Amblyopia has been associated with lower intelligence scores (Stewart-Brown, Haslum, & Butler, 1985). Some studies reveal differences in favor of myopia on verbal IQ subtests which employ reading, and no differences on nonverbal/nonreading IQ subtests (Williams, Sanderson, & Share, 1988; Young, 1963). Although the evidence strongly supports a relationship between intelligence and acuity status, these studies are possibly confounded by prerequisite reading skills needed to take IQ tests (see next section).
Achievement scores
Varying acuities have been associated with teacher ranked ability groups: high, medium, and low reading ability; and academic vs. vocational educational programs (Grosvenor, 1970). Myopia is found mostly in high and medium ability groups, and hyperopia is found mostly in medium and low ability groups. Myopia is associated with higher ability levels and academic programs. Schwartz (1938) reported that 43% of 1,000 poor readers were hyperopic. Hyperopia has links with lower achievement scores, (Rosner, & Rosner, 1997), particularly reading (Hirsch & Nadell, 1958; Williams, Sanderson, & Share, 1988), even after equating achievement scores for IQ (Stewart-Brown, Haslum, & Butler, 1985.) Because of the adverse effects of hyperopia on reading, Rosner and Rosner (1997) recommend prescription of glasses for +1.25 D or more of hyperopia. However, studies are divided on whether or not corrective lenses cause significant improvements in subjects' reading scores (Stewart-Brown, Haslum, & Butler, 1985; Grisham & Simons, 1986).
THEORIES
Does myopia give a student a reading advantage, and consequently, an IQ advantage (Grosvenor, 1970; Hirsch & Nadell, 1958)? Differences in IQ scores may be due to ability to do near work, particularly for extended periods of time. Uncorrected myopia produced better reading scores than corrected myopia (Grisham & Simons, 1986). This may also lead to another advantage of knowledge previously acquired through reading (Grosvenor, 1970; Young, 1963). These advantages may account for higher scores on intelligence tests by children with myopia.
Does myopia cause a student to become a reader, or does reading cause myopia (Hirsch, 1964)? Genetic predispositions toward myopia could lead to the natural reinforcing of particular experiences and competencies. The myope may prefer reading, which may in turn may further degrade visual acuity, but also strengthen the ability to do near work (Grosvenor, 1970 &1971). Hyperopia may be a hindrance to efficient reading because of the effort required to accommodate (focus) on closer targets. Even moderate amounts of hyperopia can cause physical symptoms during reading, such as rapid fatigue, discomfort, and nausea (Grosvenor, 1971). The same hyperope might pass a screening test for near vision using single letters, because the time on task is not sufficient to bring about any physical symptoms.
REFRACTIVE ERRORS IN DISABILITY POPULATIONS
The research literature has few dissenters on the higher incidence of refractive errors in moderate to severe disability populations. Some specific syndromes are also known to be associated with visual impairments and/or refractive errors. In general, the incidence of refractive errors increases in association with more severe disabilities. Below are some specific disabilities and some studies conducted on populations with moderate-severe disabilities, including Down syndrome and autism.
Assessment
Disability populations have been difficult to assess because many are lacking receptive/expressive language or have behavior problems (Kirschen, 1954). Cooperation and understanding of tester directives are necessary for accurate assessment. Modified Snellen charts may use familiar outlines instead of an "E" in various orientations. Apple, House, Square, or Circle on the chart are matched nonverbally with replicas that the child can point to instead of verbalizing (LH Symbols: Measurement of Visual Acuity for Children, Hyvahyvarinen, 1991). Teller Acuity Cards, another nonverbal means of assessment, uses visual preference to determine acuity (Teller, Morse, Borton, & Regal, 1974). Photorefraction (Howland, 1985; MTI Photoscreener, Ottar, Scott & Holgado, 1995) is an easy, quick, cheap, and nonintrusive screening method, but it underestimates refractive errors compared with dark refraction by about 1 D (Howland, 1982). Some studies resort to inferring acuity by behavioral observation (Castane, Peris, & Sanchez, 1995). Cycloplegic and noncycloplegic assessments are not well correlated at any age in disability populations (Kohl & Samek, 1988).
DISABILITY POPULATIONS
General disabilities populations
Many studies conducted on moderate-severe populations have shown higher incidences of hyperopia (45-59%), myopia (22-24%), and amblyopia/strabismus (28%) (Courtney, 1971; Castane et al., 1995; Kirschen, 1954; Kolb, 1962; Manley, & Schuldt, 1970). Although some of these studies included instiutionalized emotionally disturbed subjects along with moderate to severe disabilities, these too had higher incidences of hyperopia than Hirsch's youngest school population (ages 5-6 yrs.), which would be expected to have higher incidence of hyperopia than normal older populations (Courtney, 1971). In fact, all of the above percentages for types of refractive errors are higher than in Hirsch's study, and higher than would be expected in the normal population (M. Knowlton, instructor, Univ. of MN, class on photorefraction, October 1998).
Specific disability populations.
Contraction of reubella during pregnancy has been associated with increased incidence of severe myopia, cateracts, and glaucoma (Cooper, 1969). Fifty percent (50%) of children with cerebral palsy, 25-33% of children with hydrocephally, and 80% of children with myelodysplasia are estimated to have vision problems (Jackson & Vessey, 1996). Down syndrome populations have a high frequency of myopia (23%), hyperopia (22%), amblyopia/strabismus (57%), and cataracts (11%) (Caputo, Wagner, Reynolds, Guo, & Goel, 1989; Courtney, 1971).
Studies conducted on subjects with autism do not seem to indicate problems with acuity (Janicki, Lubin, & Friedman, 1983). However, this may be an artifact of a lack of comprehensive screening, due to the fact that these children are difficult to assess. Children with autism exhibit behavior which is indicative of visual problems, such as lack of visual responsiveness, squinting, scrutiny of detail, use of peripheral vision, head tilting, reliance on near space, lack of fixation on targets in far space, and posture and motor coordination problems (Bourgeois, 1971; Kaplan, Carmody, Gaydos, 1996; Kohen-Raz, Volkmar, & Cohen, 1992; Schulman, 1994). Use of photo-refraction may help to give a clearer picture of acuity for children with autism. In some cases, Vision Therapy has been used to modify the visual behavior of affected children (Kaplan et al., 1996; Rose & Torgerson, 1994; Schulman, 1994).
Treatments
Treatment of refractive errors with corrective lenses is a logical way to improve visual functioning. Glasses are usually prescribed to be optimal at 20 feet, which may compromise classroom functioning (e.g., near work) (M. Knowlton, Ph.D., University of Minnesota, class on photorefraction, October 1998). However, because much of the optometrists/ophthamolgists procedures require extensive cooperation, it is possible that clients with low verbal and/or behavioral skills will not be accurately assessed. It may be that the higher functioning a subject is, the more likely s/he will be prescribed an appropriate set of corrective lenses. If the client cannot give feedback on lack of improvements that glasses have made, s/he must depend on teachers or parents to infer acuity from behavioral cues. Furthermore, errors of correction may result if the client does not wear the glasses properly, or if the frames become bent (S. Endris, itinerant vision teacher, December 10, 1998). A simple way to test the prescription of corrective lenses is to use photorefraction. Appropriate prescriptions will cause the eyes to appear emmetropic. Often refractive errors go uncorrected because the child refuses to wear glasses (Sally Endris, itinerant vision teacher, personal communication). Other times refractive errors are undetected because a cognitive deficit masks visual problems (Sally Endris, vision itinerant teacher, personal communication). For instance, a hyperopic student may just look like s/he is not able to pay attention, when they are uncomfortable focusing on targets which are too close.
Theories
Why is there a higher incidence of refractive errors in disabled populations? Are IQ or achievement related to refractive errors? It could be reasoned that the correlations of IQ/achievement and refractive error were attenuated on the lower end of ability because those students were not included in the studies. If the average and gifted populations were combined with the subaverage intellectual populations, it seems logical to think that stronger correlations would be found for hyperopia and lack of intelligence or achievement. Even among the moderate-severe populations, it has been stated that more severe visual problems were found in subjects with lower IQs (Courtney, 1971; Kirschen, 1954). Severe visual impairments can restrict perceptual information which is important for development, especially in populations with severe disabilities (Ferrell & Raver, 1991; Hatton, Bailey, Burchinal, & Ferrell, 1997). Perhaps this is also true of refractive errors in disability populations.
Frequently mentioned in these studies is the history of birth injuries as a possible explanation for refractive errors (Courtney, 1971; Kirschen, 1954; Manley & Schuldt, 1970). Trauma to the brain and/or nervous system would affect the eyes as a part of that system. Both the brain and the eyes are more highly developed than other body parts, and have less growth to accomplish after gestation (Courtney, 1971).
Additionally, refractive errors could be caused by a lack of physiological development. Many developmental disabilities are accompanied by a lack of growth, which may also affect the brain and/or the eyes (Courtney, 1971; Hirsch & Nadell, 1958). Retarded development of the eyes could explain the higher incidences of hyperopia, which are also found in young children. Also, reports of "exaggerated" responses to cycloplegia in disability populations is reminiscent of early development (Manley & Schuldt, 1970).
CONTRIBUTIONS OF VISION TO ACQUISITION TO ACQUISITION OF VERBAL AND GESTURAL LANGUAGE
eOther than literature on development of blind children, little research can be cited that directly addresses the effects of vision on language development. In the following section, research on blind, delayed, and normal development are brought together to suggest possible relationships between vision and language development. Additional comments are made about social factors which facilitate language.
Selected Language Features
Joint attention
Implicitly or directly stated, facilitation of joint attention is a necessary component of language intervention. Joint attention measures have been associated with language measures (Mundy, Sigman, & Kasari, 1990). Some interventions may focus on the mere sharing of affect (Greenspan & Wieder, 1998), because positive affective experiences are associated with joint reference, rather than requests (Kasari, Sigman, Mundy, & Yirmiya, 1990). Requests are easily engineered by structured milieu techniques. But more unstructured responsive interaction interventions may be more likely to stimulate shared positive affect through encouragement of declarative functions (Salmon, Rowan, & Mitchell, 1998).
Even though other cultures demonstrate that prelinguistic diectic gestures are not necessary for language development, it may be advantageous for preschoolers with disabilities to be taught these skills. Because verbal language is often severely delayed in preschoolers with disabilities, it is beneficial for them to learn other methods of effective communication. Until symbolic communication develops, diectic gestures may nurture motivation for communication. However, these interventions are rarely mentioned (brief outline in Dijkxhoorn et al., 1996; one line in Rossetti, 1991; Warren et al., 1993).
Perhaps it is possible that the experience of affective sharing is "dampened" by the a mismatch between interaction distance and refractive error. Hyperopic students may not enjoy close encounters because it is too difficult to focus on the communication partners face. Myopic students may miss out on affective encounters because interaction distances are too great. Mismatches may produce less positive affect and lead to lower motivation for communication.
Diectic gestures
Visual requirements are inherently involved in the comprehension of gestures. They must be seen to be communicated. Congenitally blind children do not acquire diectic gestures without direct instruction (Rowland, 1984). Is it possible that refractive errors in developmentally delayed children could reduce the saliency of visual information contained in gestures? At a distance, myopes might not interpret a pointing gesture differently from a reach. If diectic gestures are not clearly seen and comprehended, they may not be imitated and produced. The presence of amblyopia/strabismus may also interfere with the production of gestures because of associated fine and gross motor problems (Slavik, 1982). Additionally, is it possible that reduced saliency of environmental targets could hinder the development of communication via diectic gestures? If the diectic referent is not clearly seen, the purpose of the gesture may be misunderstood.
Symbolic gestures
Symbolic gestures may be spontaneously produced or taught to the child (Acredolo & Goodwyn, 1988). If they are spontaneously produced, they are likely to be based on motor movements that are used with referents. Research with blind children reveals that they make motor gestures associated with favorite toys at around 5 months (Fraiberg, 1977). Although these are not symbolic, they have the potential of becoming so through reinforcement. If these motor movements could be identified in normal or delayed development, responsive intervention might maintain them through reinforcement until they eventually became referential symbols.
If gestures are taught directly to the child, the motor movements could be modeled or directly manipulated (Reich, 1978). Hand over hand produced motor movements may prove especially salient to the hyperopic child. In this way the communication interventionist might overcome the problems of being too close to be seen by the child, or too far away to be salient. Hand over hand may also aid the child with amblyopia/strabismus in production of gestures, which may require fine motor skills.
Words
Studies on children who are legally blind, who are without mental impairments, indicate that they develop more slowly on language and social measures in the first years of life than partially sighted or normally developing children (Ferrell & Raver, 1991; Hatton, Bailey, Burchinal, & Ferrell, 1997). Language and developmental domain gains are directly proportional to the amount of useable vision. In cases of concurrent cognitive or developmental deficits, gains are more depressed. Is it possible that mild visual impairments (e.g., refractive errors) could also depress the developmental gains of preschoolers who have moderate to severe disabilities?
Phonetic production of verbal language may also utilize the visual field. In normal development, speech sounds which are more visible (e.g., "m," "b," & "f") make up 33% of a child's early verbal vocabulary (Vihman, in press, cited in Goodwyn & Acredolo, 1993). Children who are congenitally blind produce these sounds in significantly smaller amounts, and blind children make more phonetic errors of substitution (Mills, 1983). Refractive error could obscure these visual models of speech production. Concomitant disability and refractive error may create barriers to phonetic imitation.
Comprehension
Comprehension of early verbal language is normally very dependent upon the visual field. If a child cannot clearly see what is being referred to, comprehension, acquisition, and production may be inhibited. Refractive errors could "dampen" environmental stimuli and hinder word acquisition via the meaning mapping processes. Congenitally blind children eventually use other sensory abilities to form understandings about the meanings of words. However, words are slow to generalize to other contexts in blind children's language (Dunlea, 1989). The blind child's first words are more context bound than normal children's words and, thus, are lacking in symbolic power. Most of blind children's word extensions (generalizations to other contexts) are made on the basis of tactual similarities (Dunlea, 1989). The tactile field of perception is not as instantly available to an infant as the visual field. It takes greater activity to explore the fewer items that are available for tactile exploration. Because sight is the most efficient mode for processing environmental stimuli, it is beneficial for children with disabilities and refractive errors to make the best use of their vision. Interventionists who are sensitive to the effects of children's refractive errors might arrange instruction and the classroom environment to enhance visual function.
Because children with developmental disabilities are slow in their physical maturation, they may be slow to integrate information from various sensory channels, due to the presumed retarded neurological maturation. Interventionists might need to consider the assumptions made when communicating. Prompts and responses might need to be established in one mode (e.g., gestural or verbal) before presuming that cross-modal responses would take place, whether or not simultaneous input from another mode is given. This also implies that the interventionist would model the mode that the student is to respond in, which may facilitate acquisition. Additionally, use of redundant gesture+word combinations by the interventionist may also facilitate comprehension for students with cognitive disabilities, provided the refractive errors and distances are compatible.
LANGUAGE RELATED FEATURES
Reading
Reading has implications for the development of language. For this reason, speech-language pathologists are now considering the various types of language that are involved with academic work (Butler, 1995). Although not every child with a disability will learn to read, they are usually expected to engage in activities which involve books, worksheets, or computers at near distances. Additionally, the emphasis on mainstreaming will bring children with disabilities into regular education classrooms where close seat work is the norm. Books, worksheets, and computers become the referents to which language refers. If children with disabilities are to be fully integrated, and to get the most (language learning) out of their mainstream experiences, the referents should be discernable. Corrective lenses may aid significantly in the quality of these encounters. However, the 20 feet standard distance correction may not produce positive effects for near work (approximately 2 feet). If glasses are not used, the teacher could be sensitive to the child's visual needs by restructuring an activity. For instance, a child may not engage with books which are presented at close distances, but may do so when stories are presented on a felt board at a greater distance.
Social Aspects
Social development may also develop more slowly for children with refractive errors and concomitant disabilities, because pragmatic cues may be obscured by refractive errors (e.g., facial expressions or environmental cues). Children who are congenitally blind socialize one-fourth as much as their sighted peers, and are retarded on measures of social development (Dote-Kwan & Chen, 1995). Children with concomitant blindness and cognitive disability are delayed socially even more (Ferrell & Raver, 1991; Hatton et al., 1997). Is it possible that refractive errors may additionally limit the social development of children with moderate to severe disabilities, in a similar manner? If a child is unresponsive to pragmatic cues, communication partner input may change (McArthur & Adamson, 1996). While this may serve an adaptive function, it can also serve to limit the richness of the social/language model, reducing the likelihood of exposure and production of more mature communication (McArthur & Adamson, 1996).
Social factors interact in complex ways throughout development (Hewitt, 1998; Sameroff & Chandler, 1975). A child that gives unreadable communicative signals to potential partners may initiate a cycle that sustains that breach. Caregivers may be less motivated to communicate with the child, and so fewer communication opportunities are utilized. If intervention is attempted, restrictive methods designed to produce desirable behavior or eliminate undesirable ones may alienate the child. Instead of learning the joys of communication, they are actively avoiding it. Similarly, a child that is motivated by attention may initiate a cycle of chasing their communicative partners. Interactions with peers may be less manipulable than those with adult interventionists. Because of the important of social competence with peers, corrective lenses may be the most facile vision enhancing ploy. Corrective lenses are not usually recommended at young ages for hyperopia, but are more commonly recommended for myopia. Depending on the severity of refractive errors, this may not pose a significant barrier. Mildly hyperopic children will see the social environment well in group settings. Peer attitudes toward children with disabilities can be positively affected through interventions, but may take significant teacher time in monitoring to remain effective (Lerner, Lowenthal, & Egan, 1998).
INTERVENTIONS
Early Childhood Special Education (ESCE). Intervention in the ECSE classroom is greatly concerned with the development of language. The techniques used to remediate language deficits are designed from theories of social interaction (Lerner et al., 1998). Classrooms are designed and programmed to facilitate high levels of language and social interactions between peers. Classrooms that use best practices more frequently also demonstrate greater language gains among the children that they serve (Schwartz, Carta, & Grant, 1996). However, a restricted range of language intervention styles were used for students with moderate-severe disabilities (Schwartz et al., 1996). Most frequently, the students with moderate to severe disabilities were the focus of language intervention, instead of other objects, events, or people. Interaction usually took place in 1:1 settings, rather than in small or large groups. Taken with information on refractive error, this means that preschoolers with disabilities, who are likely to be hyperopic, are likely to be interacted with at close distances. That the interaction is probably at close range, may partially compensate for this mismatch by increasing the saliency of the interventionist (e.g., the interventionist will take up more of the field of view).
It should not be ignored that children are also capable of compensating for their lack of visual ability. Even children with severe disabilities may make good inferential use of remaining vision. Students with severe hyperopia may rely more on touch and auditory cues when an interventionist is interacting at close range with them. They may even produce the behaviors that the interventionist is looking for. However, the issue here is to optimize language interventions by making the best use of the learner's visual abilities, as the visual sensory mode is the most efficient for quickly processing visual information. If optimal use can be made of vision in the language intervention context, it seems that this would enhance interventions. Perhaps the removal of visual cognitive-processing barriers ought to liberate cognitive space for language learning.
Assessments for ECSE are primarily concerned with the developmental age of the child. Vision screening is employed in ways that do not involve distance acuity (D. Ornelas, school nurse, personal communication, December 1, 1998). Even if hyperopia was identified at preschool age, it is not likely that corrective lenses would be prescribed, as normal children's eyes are still developing toward emmetropia. This places greater burden upon the teacher in the ECSE classroom to use environmental accommodations for children with refractive errors.
Developmental disabilities
Down syndrome interventions have been sensitive to the connection between the disorder and the need for corrective lenses. Other children with developmental disabilities are not necessarily as likely to receive corrective lenses. Often, cognitive disabilities mask visual problems (S. Endris, itinerant vision teacher, personal communication, December 10, 1998). Whether or not glasses are prescribed, intervention can take information about refractive errors into account. Interventions for children with cognitive deficits often relies on making important information salient to the student. Optimizing intervention for students with hyperopia might emphasize communication at a distance. Participation in large motor activities contingent upon verbal or gestural communication might include being thrown a large ball, running to a distant target, or jumping into a pool. Other language targets, such as locatives, are easily woven into motor activities. If near space had to be used with a hyperopic child, utilizing visual information of a broad nature could be used (as opposed to detailed information). For instance, large crayons and bold outlines could be used for coloring worksheets in an activity where children are to verbalize what they are coloring and which colors they are using. Optimizing the intervention for a child with myopia might emphasize use of near space, which is very natural in 1:1 exchanges. Using interaction contexts at greater distances might involve use of a social interaction song. When the child who is "it" calls out the name of the next child, all the children can repeat the name and point (redundant word + gesture) to the next person. When the teacher works with a child with amblyopia, additional considerations should be taken to favor the better eye during interactions, especially if the teacher sits next to the student. Some children with amblyopia use one eye for near distances and the other for far distances (M. Knowlton, Ph.D., Univ. of MN, class on photorefraction, October 1998).
Autism
Autism intervention often relies heavily on visual mode to communicate important information. Although there is no current research which links autism and refractive errors, visual deficits should be ruled out in order to ensure optimal intervention effects. It may be that cognitive factors prevent children with autism from functional use of their vision (e.g., attention to stimuli, processing of stimuli, cognitive implications of stimuli). Any additional refractive errors can only exacerbate learning problems through the visual mode. Optimizing interventions for children with hyperopia might mean use of color coding, bold lines, or texture schedule icons. (Visual schedules are often used for autistic preschoolers to establish child expectations about classroom activities.) Social Stories, a popular book technique for establishing expectations of child behavior, might need to transferred to the felt board. Optimizing interventions for children with myopia might involve large wave gestures when greeting the children from a distance (word+gesture). If a video monitor is to be watched from some distance, the room could be darkened, to make the image more salient.
SUMMARY
The saliency of the visual information connected with gestural or verbal referents is somewhat dependent upon the refractive state of the child's eye. Language or social development is dependent upon the processing of relevant symbols and social or environmental cues. Children who have cognitive or social impairments and, thus, are prevented from acquiring presymbolic or symbolic communication may benefit from more efficient use of available visual information. Disability populations have a higher incidence of refractive errors than that found in normal populations. Language remediation, which is often done at close interaction distances, may be too close for hyperopic students. Those students with myopia may not be able to discern distant environmental features which are critical to language learning. With respect to the specific needs of children with moderate to severe disabilities, we do not know the degree to which refractive errors exacerbate their developmental delays. A variety of interventions exist and may be tailored to their needs. These may take the form of corrective lenses, environmental arrangement, and/or teacher engineered interactions. However, these interventions have not been studied sufficiently with developmentally disabled children who also have refractive errors. Clearly, much work remains to be done.
Based on this review of the literature, this study was designed to examine several relationships between language and visual status in young children with and without disabilities. Participants with disabilities of the chronological ages of 3-5 years were compared with a sample of children without disabilities who had the similar cognitive levels and chronological ages of 1½ - 2½ years. The study planned to measure participants' (1) ability to learn novel words (language mapping), (2) frequency of visual targets (e.g., looking at the speaker's face and/or direction of gaze), and (3) visual acuity. Furthermore, analysis was planned for participants' relationships between (4) acuity and looking, (5) looking and language mapping, and (6) acuity and language mapping. Research for each of these six topics are described below.
1. Would participants with disabilities differ from typical children of comparable cognitive ability in terms of their ability to learn novel words? Would participants with specific disabilities, such as Down syndrome or Autism, have greater difficulty with novel word learning? Are higher developmental levels associated with higher accuracy in novel word learning? When participants are accurate in learning novel words, are those with higher developmental levels more likely to integrate vocalizations in their nonverbal responses?
2. Would participants with disabilities differ from their "cognitive comparisons" without disabilities in terms of their frequency in looking at visual targets? Would different visual targets (e.g., the speaker's face or the speaker's direction of gaze) be associated with different profiles of looking for participants with and without disabilities? Would participants with specific disabilities, such as Down syndrome or Autism, produce differences in the amount and types of looking behavior? Are higher developmental levels associated with higher frequencies of looking? Do specific interventions, such as shifting eye gaze, head turns, or use of hand gestures, facilitate looking frequency? Is the intervention effectiveness associated with participant characteristics, such as cognitive ability or documented disability?
3. Would participants with disabilities differ from their cognitive comparisons without disabilities in terms of their visual acuity? Would participants with specific disabilities, such as Down syndrome or Autism, have different visual acuity profiles when compared with participants without disabilities? Do specific interventions, such as shifting eye gaze, head turns, or use of hand gestures, facilitate language learning? Is the intervention effectiveness associated with participant characteristics, such as cognitive ability or documented disability?
4. Would participants with disabilities differ from their cognitive comparisons without disabilities in terms of the relationships between visual acuity and visual target frequency or type? Would participants with specific disabilities have different relationships when compared with participants without disabilities?
5. Would participants with disabilities differ from their cognitive comparisons without disabilities in terms of the relationships between visual targets and language mapping? Would participants with specific disabilities have different relationships when compared with participants without disabilities?
6. Would participants with disabilities differ from their cognitive comparisons without disabilities in terms of the relationships between visual acuity and language mapping?
The answers to these questions may yield information which is useful for language intervention design for children with disabilities. Language intervention for these children may assume certain language sub-skills (e.g., visual acuity or language learning strategies) which may be similar, more frequent, or deficient when compared to children without disabilities. If the specific information alluded to in the above questions is known, language intervention might become more accurate and effective for children with disabilities.
CHAPTER 3
METHODS
This study examined the visual acuity and looking behavior of participants with and without disabilities in the context of a fast-mapping language exercise (novel word learning). A modified version of Baron-Cohen et al.'s (1997) warm-up procedures with familiar toys were used to support inferences of the children's demonstration of their knowledge of novel words. Children were asked to identify familiar toys in order to validate the use of a similar procedure to test whether or not fast-mapping had occurred. Baldwin's (1993) procedures for novel word learning were used to compare the effects of visual targets (e.g., speaker and speaker's direction of gaze). Under naturalistic-like conditions, the researcher uttered a novel word five times while looking at a novel object (held by the child or the researcher). Additional intervention trials which used eye gaze shifting, head turns, and hand gestures were administered to facilitate performance on looking targets and fast-mapping for participants who did not successfully map novel labels. To determine whether novel word learning had occurred, participants played a "find-it" game in which the novel objects were hid temporarily under a towel. Finally, visual acuity was assessed with a photo-refraction camera to determine the presence and degree of vision problems.
PARTICIPANTS
Participants were chosen from Early Childhood Special Education Programs in the Twin Cities Metro and Suburban areas. Seven school districts agreed to participate in the study, and teachers within these districts were asked to nominate children 3-5 years of age who were functioning developmentally at half or less of their chronological age. A control group was drawn from typical children from a church nursery program who were approximately 1½ - 2½ months of age.
After teachers nominated participants for the study, consent forms (see Appendix A) were sent home to parents explaining the study and inviting them to allow their children to participate. For participation in the study, parents would receive the results of their children vision screening, which could aid in referrals for optical or medical services. If parents agreed, the vision screening results would be placed in the child's educational file.
The number of forms that were sent home with parents (ECSE programs) or mailed (church children's program) to parents was 146, 106 in the public schools and 40 in the church children's program. Sixty-nine (69) forms were returned, 42 from public school student guardians and 27 from the church children's guardians. Sixty (60) students were actually participated in the study, 38 from the schools and 22 from the church. The typically developing children attended a suburban church program (n = 22). The seven school districts were from both urban (2 districts) and suburban (5 districts) areas. The total of public school children from urban schools was 22 and the total of public school children from suburban schools was 16. The total for urban study participants was 22 and the total for suburban participants was 38.
MATERIALS
Six familiar toys were presented in three pairs: (1) car-shoe, (2) baby-boat, (3) ball-phone. Objects within pairs were of similar size. Eight (8) novel toys were created to be at least 5 inches long and brightly colored to ensure that far-sighted participants could see them easily. The toys were hand made from hardware store parts (knobs, colored string, rings, etc.) to ensure that the subjects would have never seen the toys before. The novel toys had simple, one-step functions similar to Baldwin's toys. A list of the supporting equipment used in this study is as follows: card table (.91 meters X .91 meters), high chair, white tablecloth, white towel (for find-it game), large popcorn tin for familiar toys, camcorder, MTI photo-refraction camera (Ottar, Scott & Holgado, 1995), and large sheets of paper to block out light from windows (use of the photo-refraction camera requires a darkened room).
PROCEDURE
Data collection took place at the educational or church sites. In the schools, children were taken to another room within the school building when school was in session (e.g., occupational therapy room, empty classroom). In the church, children were taken to an empty nursery room when children's programs were in session.
Upon entering the testing room, participants were shown a brightly colored popcorn tin on a square folding table which was draped with a white table cloth. The children's interest in the tin was piqued through various means: (1) drumming on it, (2) asking them to pick it up, (3) exclaiming about the characteristics of the tin, etc. When the children were sufficiently interested, they were seated in a high chair in front of the table. The tin was then emptied of its contents, the familiar toys. The child was allowed to investigate the items, while the researcher commented on the items, readied the camcorder, and positioned herself across the card table from the child.
To determine the validity of the novel word testing procedures, a modified version of Baron-Cohen et al.'s (1997) "warm-up" activity was conducted with the participant. If the participant could correctly identify familiar toys in response to a verbal prompt, it was reasoned that they might be able to do the same for a novel toy, whose label had just been learned. The warm-up activity was conducted using the familiar toys (see materials). Three pairs of toys were presented serially to the participant. While the first pair was hidden under a towel, a song was sung ("Gonna Find It," see Appendix F). When the towel was removed, the researcher asked, "Where's the [familiar toy]?" If children reached for, picked up, touched, or pointed to the correct toy, the researcher cheered the child's response. If no response was made, the researcher modeled the correct response by holding up the correct toy and cheering the child (as if s/he had made the response). During videotape coding, only child-initiated correct responses were credited as correct. Sometimes the child added a verbal or vocal remark to his/her gestures or manual choices. These were also noted during the coding of the video tapes to answer questions about integration of vocal and gestural modes.
Phase One of the fast-mapping exercises were similar to Baldwin's (1993) procedures. Each child was presented with two novel toys, one of which became the child's and the other became the researcher's. In one condition, the researcher would utter a novel word in verbal context ("It's a toma.") while looking at the child's toy (follow-in condition). In the other condition, the researcher would utter the novel word while looking at the researcher's toy (discrepant condition). The novel word would be said when the child's attention was directed to the child's toy, and the researcher would continue to look at the toy of focus for four seconds.
After five utterances using the novel word, the researcher would take both toys and place one toy on the right side of the table and the other on the left. To determine if novel word learning had occurred, the researcher would ask the child to identify the novel object of focus (e.g., "Where's the toma?"). The researcher would look at the child's face only. Child replies were acknowledged in a neutral manner ("Oh - O.K."). After the child answered (or did not answer) s/he was asked again to identify the novel object ("Let's do that again."). If the child answered correctly both times, the researcher would play the find-it game (similar to the warm up activity) with the child (described below).
If the child answered incorrectly or ambiguously, the researcher would perform an eye gaze shifting intervention. This was done by first causing the child to look at the researcher's face (say the child's name and/or make gestures). Then the researcher would shift her eye gaze to the correct object and say, "Look at the toma!" Eye gaze shifting intervention was done twice. Then the child was again asked twice to identify the location of the novel toy.
Next in the protocol was the find-it game, which was similar to the warm-up activity. The two objects were placed under a towel in reverse orientation and the song "Gonna Find It" was sung. When the towel was removed, the child was asked to indicate where the novel object was ("Where's the toma?"). The game was repeated again after the researcher had switched object positions again. Toys were held beyond the reach of the child during the interventions and novel language prompts All participants received the follow-in and discrepant conditions. To aid in the management of children's attention, a song was sung to introduce the new toys for each condition ("New Toys Comin' Up," see Appendix F).
Phase Two tests were additional conditions that were administered to children who were not successful in mapping the novel words to the novel objects. These interventions consisted of a head turn condition and a show gesture condition. Like the eye gaze shifting cues in Phase One, they were intended to increase looking at target toys, and perhaps facilitate language mapping.
As at the start of other conditions, the song "New Toys Comin' Up" was sung to introduce another pair of novel toys. After the child was allowed to play with the toys, they were placed in the right-left orientation on the table and the participant was given two trials of eye gaze shifting intervention. Then the child was asked twice to identify the novel object. If the subject was correct, the language test was administered ("find it" game). If the child was incorrect, the child was given two trials of novel toy labeling intervention using a head turn or a hand gesture ("show"). The head turn intervention was included eye gaze shifting. The show gesture included a head turn and eye gaze, thus these language cues varied in salience from eye gaze (least salient), to head turn, to hand gesture (most salient). After the head turn or show gesture intervention, the child was again asked twice to identify the novel object. Then the find it game was played twice, reversing the object positions both times. A flow chart of language learning and testing procedures can be found in Appendix D.
After the language exercises, the child's vision was assessed with the photo-refraction camera to determine the presence and degree of refractive errors. Other types of vision problems, such as binocular coordination problems were also noted in the data. Photographing of the participant after language activities prevented after-images from the flash from interfering with the child's vision during the fast-mapping exercise.
During the warm-up activity the order of pair presentation of the familiar toys and the requested toy position (right or left) were counterbalanced for the disabilities and typical groups. For the experimental conditions (follow-in, discrepant, head turn, and show gesture) novel toys, novel labels, toy position were counterbalanced for the groups. Cue cards were developed to aid the researcher in the proper execution of counterbalancing (see Appendix E).
CODING AND INTERRATER RELIABILITY
Coding - Once the camcorder was recording and before the language tasks took place, "calibration" of the child's eye gaze was performed. This permitted the inference of child eye gaze targets. Participant eye gaze "calibration" was performed by directing the child through word or hand movements to look at specific places: (1) the researcher's face, (2) the researcher's play area on the table, (3) the right side of the table, (4) the left side of the table, and (5) the child's own play area on the table. The placement of the camcorder, the high chair, and the size of the table all created standard distances and angles which aided in the coding of the videotapes. Calibration helped to sort out any coding difficulties due to ocular coordination problems, where it was possible that only one eye would be focused on any coded targets.
Videotapes were coded for data during the warm-up activity, Phase One, and Phase Two. The warm-up activity data consisted of (1) correctness of responses to the six prompts to identify warm-up activity, (2) number of correct responses, (3) number of times vocalization occurred simultaneously with the correct responses, and (4) percentage of vocalization with respect to correct warm-up responses. Phase One consisted of (1) number of times the child looked in response to the five novel utterances (looks to researcher's face and sequential looks to researcher's face to direction of researcher's eye gaze), (2) percentage of the times that the child looked to these targets, (3) child choice in response to prompts to identify novel objects, (4) child responses to eye gaze shift, head turn, and show gesture interventions, and (5) responses to the find-it games.
A five point ordinal scale was used to score children's responses to identify the novel objects, the cues to look (eye gaze shift, head turn, show gesture), and the find-it games. Each of these prompts were presented twice. If the child looked both times, or chose correctly both times, the child received a score of two (+2). If s/he looked or chose once, but on the other prompt chose nothing, chose both objects, or looked at the researcher only, a score of one (+1) was given. If the child chose both items on both prompts, or chose no items on both prompts, or both on one prompt and none on the other, or looked only at the researcher's face both times, a score of zero (0) was given. If s/he chose the wrong object once and chose ambiguously the other time (both or none), or if the child looked once to the opposite toy (rarely happened) and on the other prompt looked at the researcher's face, the child was given a score of minus one (-1). If the child chose incorrectly both times, or looked to the opposite toy both times, a score of minus two (-2) was given.
Twenty percent (20%) of the videotaped data was recoded by another person unrelated to the project. Interrater Observer reliability was computed with the number of agreements divided by the number of agreements plus disagreements. Interrater observer agreement was rated at .86 for the researcher and the person unrelated to the project.
ANALYSIS
Formation of Groups for Data Analysis
Acuity Groups
An MTI photorefraction vision screening camera (Ottar, Scott & Holgado, 1995) was used to assess each subject's vision. This instrument yields highly specific information about visual function of the eyes including alignment, acuity (near-sighted, far-sighted), astigmatism, amblyopia ("lazy eye"), and presence of cataracts. The camera assesses acuity at a distance of 1.5 meters. (Typical vision screening tests for focusing ability at a distance of 20 feet.) Each photograph actually has two pictures of the participants' eyes: one made with a horizontal flash of light (horizontal axis) and the other with a vertical flash (vertical axis).
Photos were assessed in two different ways. First, each participant was assigned to an acuity group, based on the results of a photo-refraction vision screening. Based on vision in the better eye after prescriptive correction, children's photos which presented two (2) millimeters or more of hyperopia were assigned to a hyperopia group (n = 4), those with one-quarter (¼) millimeter or more of myopia were assigned to a myopia group (n = 3), those with one (1) millimeter of astigmatism, with one axis showing normal refraction were assigned to an astigmatism group (n = 5), and the rest of the participants were assigned to a normal vision group (n = 48).
The second assessment of photographs was an experimental measure of continuous data. The measured amount of refractive error for the four eyes (two pictures) was totaled and divided by two (2). Hyperopic measures received positive (+) numbers and myopic measures received negative (-) numbers (as in the optical profession). Two exceptions to this were (1) a participant which had myopia and hyperopia associated with different axes of refraction, and (2) a participant who had one eye that was totally blind and the other eye with normal vision. Because the former had greater error associated with myopia, she was assigned to the myopia group, and assigned a negative continuous data number. The latter was assigned to the normal group, but excluded from analyses based upon continuous data. The continous measure classified members of the astigmatic group between the myopia and hyperopia groups.
In addition, participants with ocular coordination concerns were noted in the data. Some of the information on binocular tracking problems was derived from the vision screening photographs, some came from teacher reports. The researcher of this study was certified by the Minnesota Department of Health to conduct vision screening and interpret vision screening photographs.
Diagnosis groups. Signed consent forms for children with disabilities gave the researcher access to educational file information. The most recent assessment summary reports contained information on the child's (1) birth date, (2) age at assessment, (3) cognitive age equivalent score, (4) expressive age equivalent score, (5) receptive language age score, (6) visual functioning information, (7) corrective prescription information (glasses), and (8) disabilities.
On the basis of disability information contained in children's educational files, three "diagnosis groups" were formed for further analysis: (1) children without disabilities ("no disabilities," n = 22, same as the "typical" group above), (2) children with disabilities except autism ("disabilities," n = 25), and (3) children with autism ("autism," n = 13). Members of the autism diagnosis group were members of the high, middle, and low cognitive groups. However, most of the low cognitive group was comprised of children with autism (10/13).
Cognitive groups
In addition to the acuity groups and the diagnosis groups described above, children were assigned to "cognitive groups" based on their mental age equivalents for the cognitive tests found in the assessment summary reports. Although standard scores would have been the preferred method of assigning children to groups, there were several difficulties that arose when this was attempted. Age equivalents were used for the following reasons: (1) The norm samples used for standard scores were typically different across ages (a different norming group for every two month increment). This prohibited the comparisons of children in the study according to standard scores, because their ages spanned several years. (2) Standard scores are accurate measures within one or two standard deviations of the normative average. Half of the children with disabilities in this study were functioning below three standard deviations when compared with their normative samples. Therefore, accuracy of the measure is compromised. (3) The month equivalents found in educational files were more easily interpreted than standard scores. They also provided a concrete measure that teachers with knowledge of child development could easily use in an accurate fashion.
The children with disabilities were placed into three cognitive groups based upon age equivalent cognitive scores. The month equivalents were divided by the child's age at the time of assessment to form a "cognitive index," a number between zero (0) and one (1). This index was multiplied by the child's age at the time s/he participated in this study to yield a "cognitive age progression." This procedure assumed that the child's functioning level would currently be the same proportion of their chronological age as it was at the time they were tested for the Assessment Summary Report. About half of the disability groups' cognitive indexes were based upon file information that was less than six (6) months old (n = 20). The other half was based upon information that was under 12 months (n = 7) and older than 12 months (n = 11).
Children in the typically developing group were assigned cognitive indexes of one (1.0), which yielded cognitive age progressions equal to their age at participation in the study. (Cognitive tests scores were not available for the typical children.) The typical children were assigned to a "typical" group. The average age of the typically developing children was 23.7 months, and the ages ranged from 16.9 months to 32 months.
The children in the disability group that had cognitive progressions that fell within the range of the ages of the typical children were assigned to a "middle" cognitive group, because their estimated cognitive ages were similar (n = 13). The typical and middle groups were used as "comparison groups," rather than "matched groups," because the cognitive tests had not been administered to the typical children. The average cognitive age progression for the moderate group was 24.3 months, and the cognitive progressions ranged from 17.78 to 31.56 months. The average chronological age was 51 months, and the ages ranged from 38.8 to 68.4 months.
Children in the disability group with cognitive progression scores higher than 32 months were assigned to a "high" cognitive group (n = 15). The average cognitive age progression was 46.4 months, and the progressions ranged from 33.2 to 73.7 months. The average chronological age was 65.6 months, and the ages ranged from 50.4 to 77.6 months.
Children in the disability group with cognitive progression scores lower than 16.9 months were assigned to a "low" group (n = 10). The average cognitive age progression was 12.9 months, and the progressions ranged from 10.3 to14.8 months. The average chronological age was 48.4 months, and the ages ranged from 37.7 to 65.6 months. Figure 1 demonstrates the chronological ages of the children that participated in the study. Figure 2 demonstrates the cognitive ages of the children that participated in the study.
Graph of the Chronological Ages of the Children in the Study
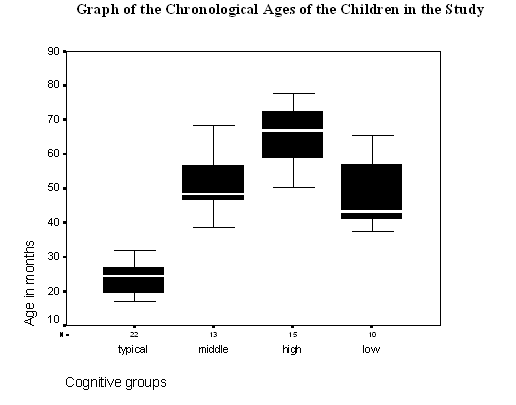
Figure 1 - Chronological ages of the children that participated in the study (by cognitive groups).
Graph of the Cognitive Age Progressions of the Children in the Study
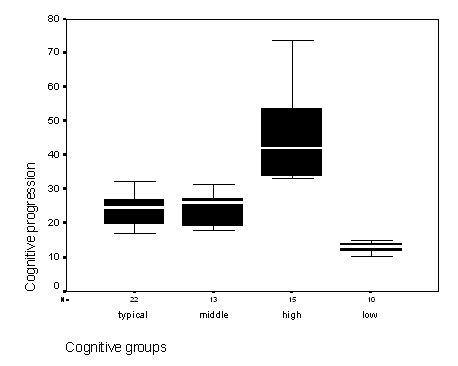
Figure 2 - Cognitive age progressions of the children that participated in the study (by cognitive groups).
DATA ANALYSIS
Data collection sheets were prepared for the photo-refraction results and the collection of file information in addition to coding information from the video tapes (see Appendix C). The data were entered into a spread sheet and analyzed with an SPSS computer software program. Pearson correlations were used to analyze relationships among assessment scores found in educational files. Spearman rank correlations were used to analyze relationships between acuity, looking behavior, and fast-mapping scores. Cognitive, Diagnosis, and Acuity group differences were analyzed with Kruskal-Wallis and Mann-Whitney tests. Where applicable, Wilcoxon related measures tests were used to determine whether interventions were effective for specific groups or the entire study sample in general.
CHAPTER 4
RESULTS
There were six major areas of data analysis: (1) language mapping of the novel words during the language tests, (2) frequency of looking during language learning, (3) visual acuity, (4) relationship between acuity and looking, (5) relationship between looking and mapping, and (6) relationship of acuity and mapping. Questions were answered by analyses which used cognitive groups, diagnosis groups, and acuity groups (see Chapter 3: Methods).
Age equivalent scores from the educational files were used to form cognitive and language indexes and cognitive and language age progressions. Indexes, a number between zero (0) and one (1), were formed by dividing the month equivalent test scores by the child's age at the time of assessment. Age progressions were formed by multiplying the index by the child's age at the time s/he participated in this study. This procedure assumed that the child's functioning level would currently be the same proportion of their chronological age as it was at the time when they were tested for the child's file information. These scores were used in correlational analyses below. The file indexes and the age progressions for cognitive, expressive language, and receptive language were highly correlated, even after removing participants who have cognitive indexes of one (1.0) (the typical children and the high cognitive group). Indexes and age progressions are used in further analyses made below. Table 1 demonstrates the intercorrelations of the progressed age measures.
CORRELATIONS OF AGE PROGRESSED FILE INFORMATION
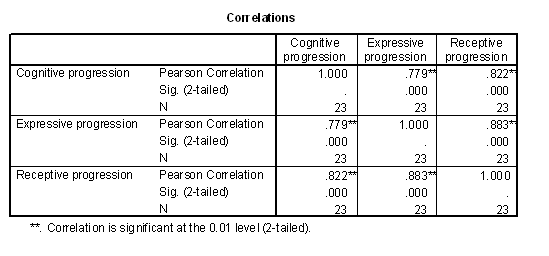
Table 1 - Pearson correlations of the progressed age measures. The typical and high cognitive groups have been removed from the analysis to prevent artificial inflation of r.
LANGUAGE MAPPING
Only participants who scored four (4) or better on the warm-up activity had data collected on mapping variables. This was done because familiar object selection which was not under the control of researcher directives made it difficult to interpret object selection of novel objects under using similar procedures. Six opportunities were given for each participant to comply with researcher's requests and four (4) or more correct responses were considered to be an acceptable demonstration of directive control. Excluding participants with warm-up scores of fewer than four (4) removed all ten participants in the low cognitive group, four in the middle cognitive group, and four in the typical cognitive group from data analysis on language mapping variables.
GENERAL RESULTS
Language mapping data were analyzed to determine if language mapping was related to cognitive or language ages. Using the entire study sample (combined groups), correlations were made for all language mapping variables and all age progressions. There were many forms of language mapping variables: participants' responses to verbal cues ("Where's the Toma?") after naturalistic conditions (follow-in & discrepant), after eye gaze shift interventions, head turn intervention, show gesture intervention, and find-it games. In addition, totals were calculated for the first two conditions (follow-in and discrepant), all four eye gaze shift mappings, and all four find-it mappings were calculated. Despite the plethora of mapping variables, none of them were related to any age progression, except for the show gesture mapping variable and expressive language age progression (rs = .52, p = .03). The lowest insignificant correlation was the show gesture mapping variable and the cognitive age progression (rs = .45, p = .06). Developmental level on age progressed measures did not seem to be related to the general ability to fast-map a novel word to a novel object for the participants tested in this study.
To test whether or not the lack of significance was related to the data collection procedures, participants' scores from the warm-up activity were also correlated with age progressions. Spearman rank correlations produced significant results between warm-up scores and cognitive age progressions (rs = .75, p = .00), receptive progressions (rs = .76, p = .00), and expressive progressions (rs = .72, p = .00). Warm-up procedures were strongly and significantly related to developmental levels. However, the find-it language mapping variables, which are similar to warm-up activities, were not related.
Language mapping data were also analyzed to determine if cognitive or diagnosis subject characteristics were associated with the ability to fast-map (learn novel words for novel objects). All of the Kruskal-Wallis tests for differences in language mapping variables were insignificant for cognitive based groups. The variables which came closest to statistical significance were follow-in find-it, show gesture mapping, and gesture find-it scores. Mann-Whitney follow-up tests revealed no significant differences for the typical cognitive group and the cognitive comparison middle disability group for any of the language mapping measures. No statistically significant language mapping differences were found for any of the cognitive groups, even though the high cognitive group was made up of children with higher cognitive age progressions than the other groups.
Most of the language mapping variables were also insignificant for diagnosis groups. The notable exceptions were significant results for the Kruskal-Wallis test of the show gesture find-it scores (p = .01) and near significance for the total find-it scores (p = .06). Mann-Whitney follow-up tests on the show find-it variable reveal significant differences between the "no disability" group and "disability" group (p = .01), and "disability" group and "autism" group (p = .02). Mann-Whitney follow-up tests on the total find-it scores reveal a significant difference between the disabilities group and the autism diagnosis group (p = .03). No differences were found for the no disability group and the autism group, which was made up of three high functioning children with autism. Figure 3 demonstrates the median differences between show gesture find-it scores for the diagnosis groups. Figure 4 demonstrates the median differences between total find-it scores for the diagnosis groups.
Graph of Median Show Gesture Find-it Scores
Figure 3 - Graph of the median show gesture find-it scores for the diagnosis groups. Mann-Whitney tests of differences is significant for no disability group and disability group (p = .01), and disability group and autism group (p = .02).
Graph of Median Total Find-it Scores
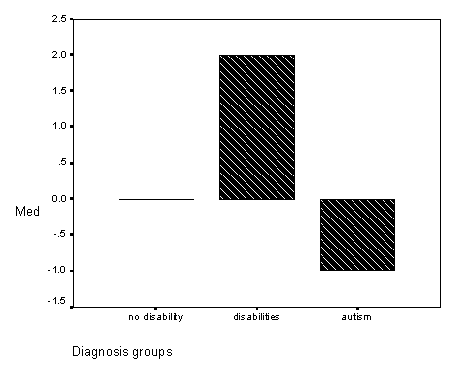
Figure 4 - Graph of the median total find-it scores for the diagnosis groups. Mann-Whitney test of differences between the disabilities group and the autism group is statistically significant (p = .03).
A case by case examination of the data was done to discover if developmental level was associated with perfect mapping scores. A record was made of all the participants who had a combined score of four (4) for follow-in and discrepant condition find-it games. The earliest cognitive age progression associated with perfect subtotal find-it scores was 23.7 months, and was found in the typical cognitive group. Two other perfect scores were associated with 27.7 months and 32 months, and were also from the typical cognitive group. In addition, two scorers from the middle cognitive group received perfect mapping scores, their cognitive age progressions were 27.3 and 29.9 months. The first perfect scorer for the high cognitive group had a cognitive age progression of 33.2 months, and the oldest of six perfect mappers in this group had a cognitive age progression of 70 months. Comparisons of the percent of perfect mappers for each group indicate that the typical group (.14) and the middle (.15) cognitive group are equivalent. Even if the frequencies are computed on partial samples using the first perfect mapper and all cognitive progressions greater than the first perfect mapper, the proportions are .23 for the typical group & .28 for the middle group. The percentage of perfect mappers for the high cognitive group was 40%.
To answer questions about vocal and gestural integration, data were first analyzed to determine whether participants who were correct in their fast-mapping choices integrated vocal and gestural modes in a similar manner to that which was recorded for their correct warm-up choices. A percentage score of vocal use during correct warm-up trials was calculated for each participant. The percent of vocal use was also calculated for each find-it condition and totaled to form a vocal score. If participants chose the correct object twice in any find-it condition, it was assumed that they were very certain of their choices. If participants were certain of choices in warm-up and in any find-it condition, their manner of demonstrating their certainty would be expected to be the same (e.g., their degree of vocal-gestural integration would be the same). Analyzing only the participants that received perfect scores in each condition separately, the Spearman rank correlations of the warm-up vocal score and the condition specific vocal scores were highly correlated in the follow-in (rs = .99, p = .00), discrepant (rs = .86, p = .00), head turn (rs = 1.0) and show gesture (rs = .89, p = .01) conditions. Furthermore, Wilcoxon and Sign tests for related samples failed to reject the null hypothesis that the scores are equal. The participants who had perfect find-it game scores demonstrated similar vocal and gestural integration in both the warm-up and find-it game conditions. Now this data could be used to attempt to demonstrate whether or not vocal and gestural integration was related to developmental level.
The coordination of gesture and speech takes place gradually, starting in the second year of typical development (see Chapter 2: Review of the Literature). Because the typical response of participants to language mapping prompts was to manually indicate the object, a simultaneous vocal addition to the response might be associated with development. With use of the entire study sample, Spearman rank correlations were computed for the three age progressions and the warm-up vocal scores, the subtotal vocal scores (follow-in and discrepant conditions), and the total vocal scores. Only the cognitive age progression was significantly related to the warm-up vocal score (rs = .26, p = .04). Analysis of the perfect scorers only (+4 for follow-in and discrepant conditions) did not improve correlations. Correlations for warm-up vocal scores were significantly related to cognitive age progressions, but none of the vocal find-it game variables were related to the progressions.
To determine whether subject characteristics were associated with differences in vocal scores, cognitive and diagnosis group vocal scores were analyzed. Mann-Whitney tests on cognitive groups revealed no significant differences for vocal scores. Diagnosis groups revealed no significant differences for vocal scores, except for the show gesture find-it vocal scores between the no disability and the disability groups (p = .01) and warm-up vocal scores for no disability and autism groups (p = .03). As with language mapping variables, few differences were found for vocal score variables.
Summary of language mapping. Few of the many language mapping variables were related to statistically significant differences or correlations except, perhaps, the show gesture mapping variables, and more rarely, total find-it scores. The case by case record of the developmental ages of perfect mappers (+4 in follow-in and discrepant conditions) did not reveal any notable trends in the age of perfect mapping or the groups from which they came from. In two analyses warm-up variables were significantly associated with progressed measures, while find-it variables were not significantly associated with progressed measures. This was the case for warm-up scores and language variables (of all kinds) and also the case for warm-up vocal scores and find-it vocal scores. Attenuation of the progressed variables may be responsible for these discrepancies. Mapping variables lack the participants in the low cognitive group, while the warm-up scores include the members of the low cognitive group.
LOOKING BEHAVIOR
Analyses of several types of looking variables (visual targets) were made to see if looking responses were related to participant characteristics. The types of looking variables were: (1) sequential looking (looking at the face of the speaker in response to a speaker's utterance and then following the speaker's direction of gaze), (2) looking at the speaker's face only (in response to a speaker's utterance), (3) looking in response to a shift in eye gaze (accompanied by a speaker's utterance), and (4) looking in response to head turn and show gesture interventions. Sequential looking and face looking were coded for the follow-in and discrepant conditions, and totals were computed. Eye gaze shifting intervention took place in follow-in, discrepant, head turn, and show gesture conditions, and totals were computed for the first two conditions and all four conditions. Head turn and show gesture interventions took place in the conditions that bore their names.
Naturalistic conditions. Looking data were analyzed to determine if looking under naturalistic conditions was related to developmental levels. The looking variables used during the follow-in and discrepant conditions (e.g., naturalistic conditions) were related to the three age progressed measures. Spearman rank correlations of total sequential looking scores (rs = .40's, p < .01) were significantly related with cognitive, expressive, and receptive measures. Total face looking scores, in comparison, were insignificantly related with the progressed measures. Total eye-gaze scores were also related, but these correlations were based on roughly half of the entire study sample and combined eye gaze shift scores from the naturalistic and intervention conditions (rs = .60's, p < .01 & .05). Table 2 gives the results of Spearman rank correlations of the progressed measures and total sequential looking, total face looking, and total eye-gaze shifting variables.
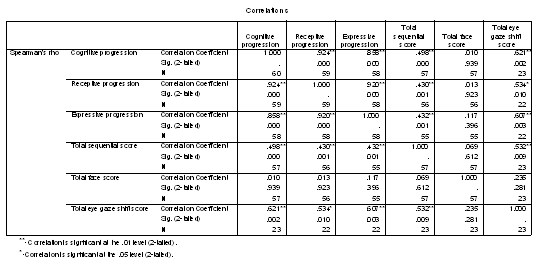
Table 2 - Spearman rank correlations of three age progression variables and three looking variables.
An additional question was formulated when data were being collected on whether participants performed more sequential looking during the discrepant condition than the follow-in condition. Correlations of sequential looking during follow-in and discrepant conditions were moderately and significantly related to the progressed measures, with discrepant measures (rs = .45-.49, p = .01) leading follow-in measures (rs = .28-.38, p = .01 & .05). A related measures sign test of the two conditions indicated that more sequential looking occurred during the discrepant condition (p = .03).
Analyses were made on cognitive and diagnosis group looking scores to see if participant characteristics were related to differences in looking scores. Kruskal-Wallis tests were conducted on cognitive groups and diagnosis groups for differences in all looking variables. For cognitive groups, all looking variables were significant (p = .01) except follow-in, discrepant, and show gesture condition eye gaze shift scores. Also, the subtotal eye gaze shift score was insignificant, but the four-condition total eye gaze shift score was significant (p = .03). (It should be noted that the total eye gaze shift score uses approximately half of the entire study sample, which may have inflated the correlation.) The many significant correlations of looking variables makes a stark contrast to the lack of significant mapping variables in the previous section.
To determine if children with disabilities differed from children without disabilities in terms of their looking behaviors, the typical cognitive group and the middle cognitive comparison groups were contrasted on all looking variables. Mann-Whitney tests for the typical and middle cognitive groups were used to test for differences in total sequential scores, total face scores, total eye gaze scores, head turn scores, and gesture scores. Only the total sequential scores were statistically significant (p = .04). Discrepant condition sequential scores (p = .07) and discrepant condition face scores (p = .06) were almost statistically significant. Graphed means show that the typical cognitive group received higher sequential looking scores than the middle cognitive group. Figure 6 demonstrates the differences in total sequential looking scores.
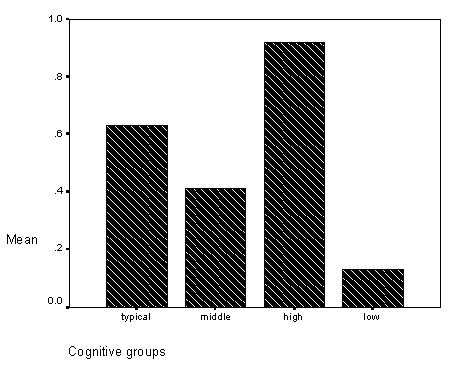
Figure 5 - Graph of the differences in total sequential scores. Kruskal-Wallis test of group differences is statistically significant (p = .00). Mann-Whitney test for typical and middle group is statistically significant (p = .04).
Analyses of diagnosis group looking scores were also made to determine if these participant characteristics might be related to differences in looking behavior. Kruskal-Wallis tests for differences in diagnosis group looking scores found follow-in, discrepant, and total sequential looking scores to be significant (p = .00). Follow-in face scores were significant (p = .04), but discrepant face scores were not. In addition, subtotal eye gaze scores were significant (p = .03), but total eye gaze scores were not. None of the intervention variables (head turn & show gesture) demonstrated significant group differences. As with the correlations of looking variables and age progressed measures, significant differences for diagnostic groups centered on sequential looking and eye gaze shifting variables. Additionally, follow-in face scores revealed differences for diagnostic groups.
Mann-Whitney follow-up tests were conducted on the diagnosis groups to determine which pairs of groups differed from one another in terms of sequential looking variables. Total sequential looking revealed significant differences between the no disability and autism groups (p = .00) and for the disabilities and autism groups (p = .00). Figure 7 demonstrates the differences in total sequential looking scores for the diagnostic groups with the use of means.
Graph of the Mean Total Sequential Looking Scores for the Diagnosis Groups
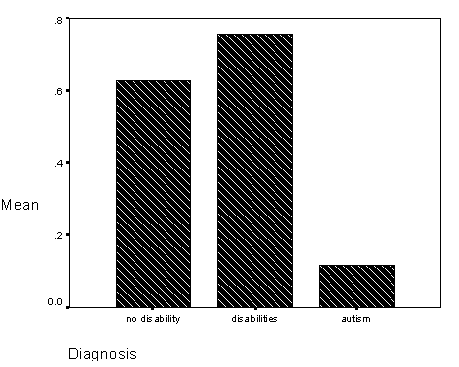
Figure 6 - Graph of the mean scores of total sequential looking scores for the diagnosis groups. Differences in no disability and autism groups are statistically significant (p = .00), and differences in disabilities and autism groups are significant (p = .00).
Mann-Whitney follow-up tests were conducted on the diagnosis groups to determine which groups differed significantly from one another in terms of their other significant looking behaviors (follow-in face looking & eye gaze shift scores). The tests on follow-in face looking revealed differences between the no disabilities and autism groups (p = .02). The tests on the subtotal eye gaze shift scores also revealed differences between the no disabilities and autism groups (p = .01), while differences were almost statistically significant for the disabilities and autism groups (p = .07). These results demonstrate that typical children perform more face looking and eye gaze shifting behavior than children with autism (in spite of differences in chronological age). They also show the tendency for children with autism to not fixate on a speaker's face or follow a shift in eye gaze direction as well as their disabled peers.
During the two naturalistic conditions, five opportunities were given for the participant to perform face looking or sequential looking behaviors (or make no response). The amount of face looking and sequential looking was compared for the cognitive groups in the follow-in and discrepant conditions. The low cognitive group performed more face looking than sequential looking in both conditions. The middle cognitive group also performed more face looking than sequential looking in both conditions. The typical cognitive comparison group performed like these low and middle groups in the follow-in condition, but the trend was reversed in the discrepant condition. More sequential looking occurred than face looking for the typical children. The high cognitive group performed more sequential looking than face looking in both conditions.
Intervention Conditions
Looking data were also analyzed to determine whether head turn or show gesture interventions increased looking scores. A Wilcoxon related measures test on the entire study sample compared eye gaze shifting scores and head turn and show gesture intervention scores for frequency of looking at language targets. The test revealed that eye gaze shifting scores differed significantly, or nearly significantly from their associated interventions (head turn: p = .06 & show gesture: p = .00). Show gesture intervention also differed significantly from head turn intervention (p = .00). A graph of mean scores for eye gaze shifting, head turn, and show gesture scores suggests that head turn obtained the lowest looking scores, eye gaze shifting scores were higher, and the show gestures scores were the highest. Figure 5 demonstrates the mean looking scores for the different looking conditions for the entire study sample.
Graph of Mean Looking Scores for Eye Gaze, Head Turn, and Show Gesture
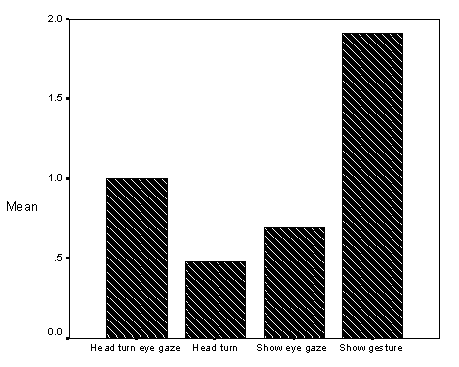
Figure 7 - Graph of the mean looking scores for different looking conditions for the entire study sample. Wilcoxon related samples tests indicate that head turn scores differ insignificantly from eye gaze scores (p = .06), and eye gaze scores differ significantly from show gesture scores (p = .00). Eye gaze scores do not differ from each other.
Analyses were conducted to determine whether head turn or hand gesture interventions increased looking behavior for the typical and middle cognitive comparison groups. These analyses used related samples tests to measure differences within each group separately. Wilcoxon related samples tests for the typical cognitive group revealed no differences between head turn scores and associated eye gaze scores, but differences were found for show gesture scores and associated eye gaze scores (p = .03). Show gesture scores were also different than head turn scores (p = .04). This presents a similar picture to that found for the entire study sample. Results demonstrate that show gesture interventions are the better than associated eye gaze shifting scores, but differences in head turn scores are not as (statistically) dramatic for the typical cognitive group.
The related samples tests of looking interventions were also conducted for the cognitive middle group. Wilcoxon tests for the middle cognitive group revealed differences for all pairs of measures: head-turn and its eye gaze scores (p = .01), show gesture and its eye gaze scores (p = .04), and head turn and show gesture scores (p = .01). These results demonstrate that the middle cognitive group differs from the typical cognitive group in the degree to which head turn interventions were effective in facilitating looking at language targets.
Analyses were conducted to determine whether head turn or hand gesture interventions increased looking behavior for each of the diagnosis groups. The related samples tests were conducted within each diagnosis group. Wilcoxon related samples tests for the no disabilities diagnosis group would yield the same results as the typical cognitive group (see above). Wilcoxon related samples tests for the disabilities (not autism) diagnosis group revealed near significance for the head turn intervention in relation to its eye gaze scores (p = .08), significant differences for the show gesture condition in relation to its eye gaze scores (p = .01), and significance between head turn and show gesture interventions (p = .00). This profile is much like that for the entire study sample. Results demonstrate that show gesture intervention is much more effective than head turns when compared to eye gaze scores for children with disabilities.
The related samples tests were also conducted for the autism diagnosis group. Wilcoxon tests revealed no differences between head turn and associated eye gaze scores, no differences for show gesture and related eye gaze scores (significance was close: p = .07), and significance between head turn and gesture interventions (p = .01). Results show that neither intervention differs significantly from eye gaze scores for students with autism, however, graphed means for the show gesture intervention are higher than head turn interventions.
Differences in eye gaze shift scores between high functioning students with autism and low functioning students with autism may have confounded the previous analyses. Therefore, analyses were repeated for only the children with autism in the low cognitive group. In spite of reduced power, the Wilcoxon differences tests revealed that show gesture interventions more effectively facilitated looking at language targets than eye gaze shifting cues (p = .02), in addition to the difference between show gesture and head turn interventions (p = .01).
Summary of looking variables
Looking variables were frequently associated with age progressions, especially total sequential looking scores and total eye gaze shifting scores. Also, while the mapping variables showed few associations with participant characteristics, many types of looking variables showed consistent correlations with participant characteristics. For instance, the cognitive groups differed on sequential looking and eye gaze shifting variables. Diagnosis groups demonstrated differences in sequential looking, face looking, and eye gaze shifting scores. The data also demonstrate that sequential looking occurred more often during the discrepant condition than in the follow-in condition. Additional descriptive data revealed that the amount of sequential looking relative to face looking increased as developmental level increased. Data on interventions designed to facilitate child looking at language targets indicated that the show gesture interventions were more consistent than head turn interventions in obtaining improvements in looking. The middle cognitive group made better use of head turn interventions than the other cognitive groups and the low functioning children with autism were significantly helped by show gesture interventions compared with head turn interventions.
ACUITY
General results of acuity data
Visual acuity and ocular coordination data were analyzed to determine which participant characteristics were associated with these acuity group characteristics. A crosstabs of the incidence of vision problems in the cognitive groups demonstrates that most of the refractive errors are found in the high, middle, and low groups. A crosstabs of the incidence of ocular coordination concerns in the cognitive groups shows that all problems with binocular tracking problems are also in the high, middle, and low groups. The typical group, by comparison, has few visual acuity problems or ocular coordination concerns. Table 3 gives the incidence of acuity problems in the cognitive groups. Table 4 gives the incidence of ocular coordination problems in the cognitive groups.
Table 3 - Incidence of problems with acuity in the cognitive groups.
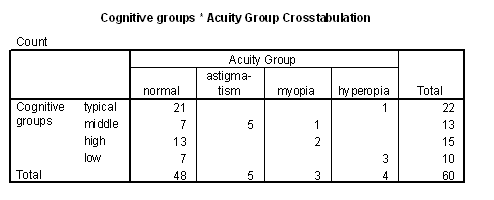
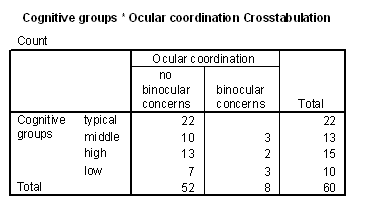
Participant characteristics represented by diagnostic groups was also examined for associations with visual acuity data. A crosstabs of the diagnosis groups reveals that most of the problems with visual acuity were found among the "disability" diagnosis group. Of particular interest is that the incidence of acuity problems are similarly low for the no disabilities and autism groups (1/13 vs. 1/22). Eight (8) participants from the entire study sample had identified binocular tracking problems (ocular coordination difficulties): strabismus (n = 6), partial occlusion (ptosis, n = 1), and total blindness in one eye (n = 1). The disabilities group also had the highest prevalence of binocular issues. Two (2) of these eight (8) participants were from the autism diagnosis group, the rest were from the disabilities group. Five (5) of the eight (8) participants with binocular concerns also had astigmatism or hyperopia. Participants with disabilities were more likely to have problems with visual acuity, ocular coordination, or both visual acuity and ocular coordination. Also, those students with disabilities that were not diagnosed with autism were more likely to have problems with visual acuity. Table 5 gives the frequencies of acuity problems for the typical, disabilities, and autism diagnosis groups. Table 6 gives the frequencies of ocular coordination problems for the typical, disabilities, and autism diagnosis groups.
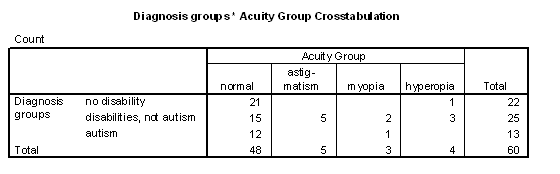
Table 5 - The incidence of visual acuity problems for the diagnosis groups.
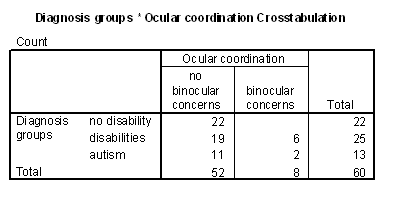
Data on acuity were recorded in three ways: (1) assignment to acuity groups, (2) the degree of refractive error (in millimeters), and (3) absolute value of the degree of refractive error (myopic scores are changed from negative values to positive values). Scatterplots of the refractive errors and file indexes (cognitive, expressive, and receptive) are similarly suggestive of a relationship between acuity and cognitive or language functioning. A scatterplot of refractive errors and cognitive indexes for the three diagnosis groups is given in Figure 8.
Scatterplot of Refractive Error X Cognitive Index for the Diagnosis Groups
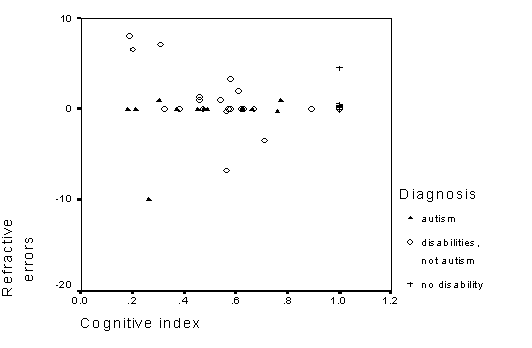
Figure 8 - Scatterplot of refractive errors and cognitive indexes for the three diagnosis groups.
To investigate whether visual acuity and developmental levels were related, Spearman rank correlations of the absolute value of refractive error were paired with the cognitive, receptive language, and expressive language indexes. Both cognitive (p = .02) and receptive (p = .04) indexes correlated significantly with the absolute value of refractive error. With the autism diagnosis group removed from analysis, whose effects tend to decrease rs with low developmental levels and normal acuity, correlations of all file indexes are significantly correlated with the absolute value of refractive error (p = .00-.02). These results show that the lower a child functions in proportion to his/her chronological age, the greater the degree of refractive error. Table 7 demonstrates the intercorrelations between the absolute value of refractive error and file indexes.
Correlation of File Indexes and Absolute Value of Refractive Error
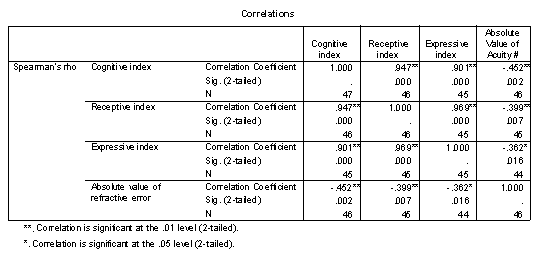
Table 7 - Spearman rank correlations between cognitive indexes and the absolute value of refractive error (millimeters of refractive error for the sum of both axes in both eyes/2). The autism group has been removed from the analysis.
Sumary for acuity data
Most of the participants with visual acuity problems were members of disability groups; they were members of the disabilities diagnosis group or the high, middle, and low cognitive groups. Typical participants and participants with autism had similarly low incidences of visual acuity problems. All of the participants with identified ocular coordination problems were also members of disability groups; they were members of the disabilities and autism diagnosis groups, and also members of the high, middle, and low cognitive groups. Five of the eight participants with ocular coordination problems also had hyperopia or astigmatism. Significant relationships between developmental indexes (cognitive, expressive language, & receptive language) and the absolute value of refractive errors were found when the autism group was removed from analyses. The same analyses which included participants with autism still revealed significant relationships with two of the developmental indexes.
ACUITY AND LOOKING
General results of acuity and looking
Relationships between acuity and looking variables were analyzed to determine whether the lack of visual acuity would affect the frequency of looking. Analyses for the entire sample using Spearman rank correlations and Kruskal-Wallis tests was not done because the presence and types of refractive errors were not balanced among the cognitive levels. (Sequential and eye gaze looking variables were related to the cognitive age progressions.) Meaningful analyses were not able to be done because tests of differences or relatedness could be attributed to cognitive functioning rather than acuity. Also, results of analyses were affected by the lack of power due to small numbers in each refractive error.
However, within each cognitive group, the acuity groups within each cognitive group were compared for differences on all looking variables. Mann-Whitney tests were used to compare differences in looking scores. For the typical cognitive group, no significant differences were found between the 21 normal children and the one (1) hyperopic child on any looking variable. Within the middle cognitive comparison group, significant differences in looking scores were found for the astigmatism group when compared with the normal acuity group in discrepant sequential looking scores (p = .05). The astigmatism acuity group performed more sequential looking in this condition than the other members of the middle cognitive group with normal vision. Total eye gaze shifting scores obtained the lowest insignificant values (p = .11). No looking differences were obtained for the one participant with myopia in the middle cognitive group. No looking differences were obtained for the two participants with myopia in the high cognitive group. No significant differences were obtained for looking variables between the three participants with hyperopia in the low cognitive group. The lowest insignificant values were obtained for the discrepant face looking and total face looking variables (p = .17), behaviors that are not habitual for low functioning children with autism. All other values were larger than .50.
Although the analyses only revealed one significant difference, that between the astigmatism group and the middle cognitive group, other differences might have been revealed with greater numbers of participants that had refractive errors. Also, the analyses for differences in the low cognitive group may be confounded because the children with normal vision in that group are all children with autism. Because the children with autism do not have high looking scores, this may minimize the effect of hyperopia on looking frequencies.
Analysis could not be done within each diagnosis group because both the disability and autism group's cognitive age progressions spanned several different cognitive groupings. This would confound the interpretation of acuity group results within each diagnosis group. Differences could be due to cognition instead of acuity.
Ocular coordination concerns and inaccurate following. During data collection, an additional question was formulated concerning the relationship between ocular coordination concerns and inaccurate following of the speaker's direction of gaze. This additional looking variable, inaccurate following, is reported here, rather than in the looking section above, because of its associations with ocular coordination issues. In addition, the inaccurate following variable has associations with inaccurate mapping, which shall be reported in this section, rather than in the Acuity & Mapping section below.
During data collection, an observation was made that participants would sometimes follow eye gaze shifting cues or head turn cues inaccurately. They would turn their gaze in the appropriate direction, but would "overshoot" the targeted object. Coding of videotapes included collection of data on this variable for all participants. Mann-Whitney tests of differences in inaccurate following scores were conducted for participants with and without ocular coordination issues (p = .00). Although participants from many different diagnosis, cognitive, or acuity groups performed inaccurate following behavior, participants that had binocular concerns had higher averages of inaccurate following than those with no binocular concerns. These differences are created by only eight (8) participants with binocular tracking problems and 52 participants without documented tracking problems. Figure 9 demonstrates the mean differences in inaccurate following behavior for participants with and without ocular coordination concerns.
Differences in Inaccurate Following by Ocular Coordination Groups
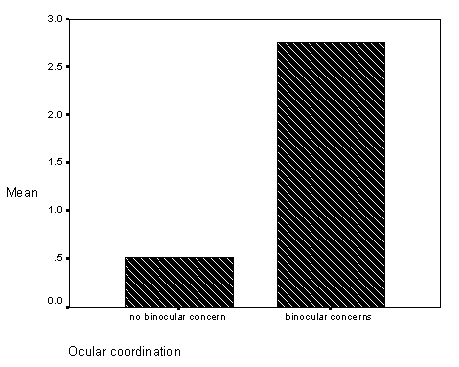
Figure 9 - Graph of the mean number of Inaccurate follows for Occular coordination groups (n = 52 for the "no concerns" group and n = 8 for the "binocular concerns" group). Mann-Whitney test of differences is significant (p = .00).
These findings are even more pronounced when considering that the mode frequency of inaccurate following for the "no concerns" group was zero (0) and the mode frequency for the "binocular concerns" group was six (6).
In addition to difficulties with matching the speaker's direction of gaze, participants with binocular concerns were more likely to inaccurately map the novel label to the inaccurate location which the participants had chosen through inaccurate following behavior. Mann-Whitney test revealed differences between inaccurate mapping scores of participants with and without ocular coordination problems (p = .03). The graph of mean inaccurate mapping scores for the ocular coordination groups is found in Figure 10.
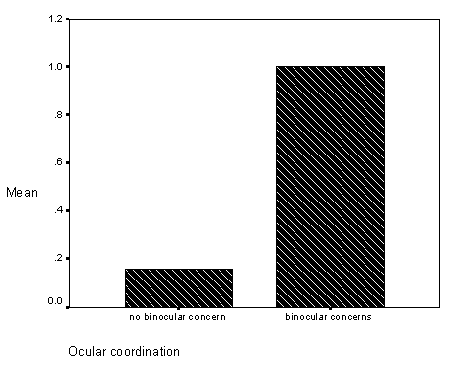
Figure 10 - The mean difference of inaccurate mapping in participants with and without binocular concerns (n = 38 for "no concern" group & n = 4 for "binocular concerns" group). Mann-Whitney test is statistically significant (p = .03).
Attempts to find differences in inaccurate following and inaccurate mapping by associations with subject characteristics did not yield any material for alternative explanations. No statistically significant Kruskal-Wallis tests were found for cognitive or diagnosis groups on inaccurate following or inaccurate mapping measures. Also, analysis was made for only those participants that performed inaccurate following. Again, no significant differences were found when comparing any cognitive or diagnosis groups on measures of inaccurate following or inaccurate mapping. The only groups that yield significant differences are the ocular coordination groups. In Table 8, a crosstabs of the frequencies of inaccurate mapping is given for each cognitive group.
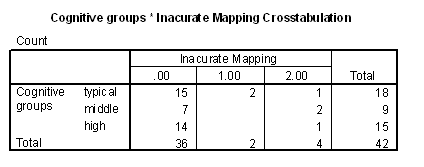
Table 8 - Frequencies of inaccurate mapping for each cognitive group (the low cognitive group does not appear due to their low warm-up scores).
Summary for acuity and looking
Analyses of the relationships between visual acuity and looking behavior were difficult because of the lack of power and the confounds with developmental levels. Each acuity group had predominant membership within one of the cognitive groups. Therefore analyses were restricted to comparisons of each acuity group to the participants with normal vision in each cognitive group. Comparisons with diagnosis groups were not possible because the same cognitive confound applied for the disability and autism diagnosis groups (these groups spanned several cognitive groups). The only significant differences for acuity group looking scores were found for the middle cognitive group on sequential looking scores during the discrepant condition. Participants with ocular coordination problems were distinguished by difficulties in matching the speaker's direction of gaze (inaccurate following during eye gaze shifting) and inaccurate mapping to the location in space where they had previously inaccurately followed.
LOOKING AND MAPPING
Questions had been asked if the frequency of looking behavior was related to the frequency of mapping behavior. To answer these questions, each type of looking variable was analyzed in relationship to language mapping variables.
Sequential looking
Sequential looking scores were examined for associations with mapping variables to determine whether the frequency of sequential looking behavior was related to frequency of mapping behavior. With the entire study sample used for analyses, Spearman rank correlations of sequential looking variables were paired with (a) follow-in, discrepant, and subtotal mapping variables, and (b) follow-in, discrepant, and subtotal find-it variables, and (c) total find-it mapping variables. The discrepant sequential condition was statistically significant with every mapping variable (p = .00-.03). The total sequential looking variable was also significantly correlated with the discrepant find-it mapping variable (p < .05). The significance values of the correlations for the total sequential looking variable were generally higher than are for the follow-in sequential variable (due to the contribution of the discrepant condition). Sequential looking, especially in the discrepant condition, is associated with mapping scores. This behavior, sequential looking in the discrepant condition, has associations beyond the context of its specific condition.
Analyses were made of sequential looking and mapping variables within each cognitive and diagnosis group to determine whether their profiles differed from that of the entire study sample. Spearman rank correlations of sequential looking and mapping variables were computed for the typical cognitive group alone. Discrepant sequential looking was correlated with subtotal find-it scores (follow-in and discrepant conditions) and subtotal mapping scores (follow-in and discrepant conditions) (p = .01 & .05). Total sequential looking was correlated with subtotal find-it (follow-in and discrepant conditions), subtotal maps (follow-in and discrepant conditions), and discrepant find-it scores (p = .01 & .05). As in the entire study sample, sequential looking and total sequential looking scores were significantly related to mapping behavior.
Examination of sequential looking and mapping variable relationships moved next to the middle cognitive group. Spearman rank correlations found that sequential looking in the discrepant condition was significantly related with the total find-it score (p = .00), however, there the similarity with the typical group ends. The other significant correlations are for follow-in sequential looking with subtotal find-it and subtotal map scores (p = .02-.05). These were negative correlations, indicating that as sequential looking in the follow-in condition increased, subtotal mapping scores decreased (rs= -.75 & -.67). For the middle cognitive group, high sequential looking scores during the follow-in condition did not appear to have a salutary effect on mapping variables. However, sequential looking during the discrepant condition does show a significant relationship with one mapping variable.
To determine if sequential looking variables were related to mapping variables at higher developmental levels, data from the high cognitive groups were analyzed. Spearman rank correlations for the sequential looking and mapping variables found no significant associations, however the lowest insignificant values were found in pairs containing the discrepant sequential looking variable. No Spearman rank correlations were computed for the low cognitive group because they had no mapping variables, due to their low warm-up scores.
Participant characteristics associated with diagnosis groups were analyzed for the same relationships between sequential looking and mapping variables. Because the no disabilities group is exactly the same as the typical cognitive group, analyses within each diagnosis group began with the disabilities group. Spearman rank correlations for sequential looking and mapping variables revealed that the discrepant sequential looking variable was significantly correlated with subtotal find-it scores and subtotal mapping scores (p = .02 & .04). However, total find-it scores were negatively correlated with sequential looking in the follow-in condition (rs = -.68, p = .02). Like many analyses above, this profile for the disabilities diagnosis group shares the significant associations between discrepant sequential looking and mapping variables. Also, sequential looking during the follow-in condition demonstrates a negative association with one mapping variable in the disabilities diagnosis group.
Completing the within groups analyses of sequential looking and mapping were analyses of the autism diagnosis group. Spearman rank correlations were computed for the autism diagnosis group alone (n = 4 mappers). No significant correlations were found. All values of rs were high, frequently negative, and associated with high significance values (p = .20-1.0). Unlike the other study participants, these high functioning participants with autism did not show a systematic association between any kind of sequential looking behavior and mapping variables.
Face looking
Even though previous analyses indicated that face looking behavior was not associated with subject characteristics (see Looking section above), analyses were performed to determine whether face looking was related to mapping behavior. Spearman rank correlations of face looking and mapping variables were computed for the entire study sample. Face looking scores in the follow-in condition were significantly associated with follow-in find-it scores (p = .02). Face looking in the discrepant condition was not significantly associated with mapping variables, nor were total face looking scores and subtotal find-it scores. Face looking during the follow-in find-it condition was significantly related to the mapping of novel labels during the follow-in find-it condition for the entire study sample.
Analyses of associations between face looking variables and mapping variables were also completed for each cognitive and diagnosis group. Within the typical cognitive group, no statistically significant association was found between face looking variables and mapping variables. Within the middle cognitive comparison group, follow-in face scores were significantly related to follow-in find-it scores (rs = .77 & p = .02). However, face looking variables were not significantly related to discrepant mapping scores and total face looking scores were not significantly related to subtotal mapping scores (follow-in & discrepant combined). Within the high cognitive group, no face variables were related to mapping scores of any kind. The low cognitive group was not analyzed in this regard due to the absence of mapping variables. In these analyses, the middle cognitive group distinguishes itself by the association of face looking and mapping in the follow-in condition.
Analysis for associations between face looking and language mapping were completed with examination of relationships within each diagnosis group separately. Within the disabilities diagnosis group, no face looking variables were related to mapping scores of any kind. Within the autism diagnosis group, no significant correlations were found for face looking and mapping, although face looking during the discrepant condition and discrepant find-it scores approached significance (p = .06). For the most part, only the middle cognitive group demonstrated significant associations between face looking and language mapping behaviors, and this happened only during the follow-in condition.
Eye gaze shift cues
Eye gaze shifting cues may have helped some participants to map when they failed to use sequential looking to fast-map novel words. Using the entire study sample to investigate this possibility, eye gaze shift variables were correlated with mapping scores directly after these looking cues and associated find-it conditions. This relationship was not significantly correlated in any of the four conditions. Also, subtotal and total eye gaze scores were unrelated to mapping and find-it variables. There was no systematic relationship between following the eye gaze shifting cues and language mapping behaviors.
Effectiveness of eye gaze shifting cues might also be measured by comparing the mapping scores before and after eye gaze interventions. Wilcoxon related samples tests and sign tests on the entire study sample revealed no statistically significant differences between mapping scores before and after eye gaze shifting cues in the follow-in and discrepant conditions. Although participants may have looked at the language target in response to the eye gaze shift cues, this does not seem to have consistently improved their mapping behavior.
To determine if participant characteristics obscured relationships between eye gaze shifting cues and mapping variables for the entire study sample, each cognitive and diagnosis group was also analyzed separately. Analyses of each cognitive group revealed that eye gaze shift intervention improved mapping scores for some cognitive groups, but not others, and for some conditions, but not others. Wilcoxon related samples tests revealed that the typical cognitive group was helped by eye gaze instruction in the discrepant condition (p = .05), and significance was marginal in the follow-in condition (p = .07). The middle cognitive comparison group was not helped by eye gaze intervention in either condition (p = .57 & 1.0). The high cognitive group was helped by eye gaze instruction in the discrepant condition (p = .04), but not helped in the follow-in condition (p = .88). The typical and middle cognitive groups differed in their ability to utilize eye gaze shift cues to more accurately map novel words. The middle cognitive group was not able to utilize eye gaze shift cues in any condition, but the typical group was able to utilize cues in the discrepant condition, while significance was very close for the follow-in condition (p = .07). Furthermore eye gaze cue significance is more consistently associated with the discrepant condition than with the follow-in condition.
Analyses of the relationships between mapping scores before and after eye gaze shifting cues continued within each diagnosis group. The no disabilities group is the same as the typical cognitive group above. Within the disabilities diagnosis group, Wilcoxon related samples tests revealed no significant differences in scores before and directly after eye gaze shifting cues in the follow-in condition. However, scores in the discrepant condition were significantly improved after eye gaze intervention when measured by find-it scores (p = .05), and approached significance when measured directly after eye gaze shifting instruction (p = .07). Within the autism diagnosis group, Wilcoxon related samples tests revealed no significant differences between mapping scores before and after eye gaze shifting interventions. Again, the discrepant condition is marked by an association with differences in mapping scores before and after eye gaze shifting cues, this time for the disabilities diagnosis group. The autism group members who map (high functioning autism) do not appear to respond to eye gaze shifting cues systematically.
As stated above, correlation of eye gaze shifting scores and mapping scores directly after eye gaze intervention for each condition were not significantly correlated when the entire sample was used for analysis. Within the typical cognitive group, eye gaze shift intervention was only correlated with mapping directly afterward during the show gesture condition (previous to the show gesture itself) (p = .02). Within the middle cognitive group, no eye gaze shifting scores were significantly associated with mapping directly afterwards. For the high cognitive group, no condition contained significant correlations for eye gaze shift interventions and mapping scores directly afterwards. Within the disabilities diagnosis group, no significant correlations were associated between eye gaze shift interventions and mapping scores directly afterward. No significant correlations were found for the autism diagnosis group, although many paired conditions did not have the required number of cases for computation. The only group that seemed to utilize eye gaze shifting cues during intervention conditions was the typical cognitive group (or the no disabilities diagnosis group), which demonstrated a significant correlation between eye gaze shifting cues and mapping in the show gesture condition.
For the entire study sample, eye gaze shift cues did not create differences in mapping scores directly before and after these cues. Eye gaze shift scores were also not correlated to mapping scores directly afterwards. Within cognitive and diagnosis groups, eye gaze shift cues were most often associated with differences in mapping scores when examining the discrepant condition. This was true of the typical cognitive group, the high cognitive group, and the disability diagnosis group. Correlations of the eye gaze shift scores and the mapping scores directly afterwards within groups were rarely significant. Only the typical group had one (of four possible) statistically significant associations. The autism group (n = 4 mappers) and the middle cognitive group had no significant associations involving eye gaze shift variables.
Head turn and show gesture interventions. Analyses of head turn or show gesture intervention scores were made to determine whether they effected differences in mapping behavior. Using the entire sample for analysis, Wilcoxon tests were conducted for differences in mapping scores before and after head turn and show gesture interventions. The tests revealed that mapping scores before and after head turns were not significantly different (p = .22), but mapping after show gesture interventions were significantly different from scores taken before the intervention (p = .05).
Analyses of each individual cognitive and diagnosis group was made with respect to differences in mapping scores before and after the head turn and show gesture interventions. Within the typical cognitive group, Wilcoxon related samples tests revealed no significant differences for mapping variables before and after head turn or gesture interventions. Show gesture intervention effectiveness as measured by show find-it scores had the lowest insignificant values (p = .10). Within the middle cognitive comparison group, no differences in before and after scores were revealed for head turn or show gesture interventions. Within the high cognitive group, tests revealed that scores improved after show gesture intervention when measured directly afterwards (p = .03). These results demonstrate that the show gesture intervention may be more effective than the head turn intervention, but only for some groups. In this case the high cognitive group and some of the typical cognitive group may have benefited from the show gesture intervention.
Analyses of differences of mapping scores before and after looking interventions was continued within each of the disabilities diagnostic groups. For the disabilities diagnosis group, Wilcoxon related samples tests of differences found significant differences for the show gesture intervention (p = .02). Within the autism diagnosis group, no before and after scores were significantly different for head turn or gesture interventions. Again, the show gesture intervention is consistently associated with differences in before and after scores. However, again, members of the autism diagnosis group fail to respond to cues and interventions designed to facilitate looking and mapping.
Summary of looking and mapping.
For the entire study sample, the relationship between looking and mapping behavior is most consistently demonstrated with the sequential looking variables, especially in the discrepant condition. The propensity to engage in this behavior, specifically in the discrepant condition, seems to be an indicator of general mapping ability. Also, face looking during the follow-in condition was sometimes significantly related to the mapping of novel labels, particularly during the follow-in condition. For the entire study sample, eye gaze shift cues did not create differences in mapping scores directly before and after these cues. Also, eye gaze shift scores were not related to mapping scores directly afterward. The study sample also demonstrated that show gesture interventions were more consistently associated with significant differences in mapping scores before and after the interventions. Head turn interventions were inconsistently significant, and sometimes had lower median scores than eye gaze shift scores.
Analyses of participant characteristics as they related to these looking and mapping relationships were conducted by examination of the individual cognitive and diagnosis groups. The typical cognitive group was much like the entire study sample in terms of its profile on sequential variables. Discrepant condition sequential looking was key to correlations with mapping variables. Face looking variables were not associated with mapping scores. Eye gaze cues helped mapping scores in discrepant conditions, and significance was close in the follow-in condition. Eye gaze score correlations with mapping afterwards were significant only in the gesture condition (previous to the show gesture). Show gesture and head turn interventions did not create differences in mapping scores before and after the intervention.
Analyses of the middle cognitive group also revealed that discrepant sequential looking was an indicator of mapping variables. But surprisingly, follow-in sequential looking was negatively correlated with mapping scores, indicating that as follow-in sequential looking increased, mapping scores decreased. Peculiar to the middle group, face looking during follow-in condition was related to follow-in mapping scores (find-it game). Eye gaze cues did not create differences in scores before and after these cues. Correlations of eye gaze scores with mapping scores directly afterward were insignificant. Head turn and show gesture interventions did not create differences in mapping scores before and after these interventions. The middle group does not seem to utilize the eye gaze cues or the head turn or show gesture interventions. Although this is not too different from the typical cognitive comparison group, there is some indication that the latter does use eye gaze cues to improve mapping scores.
The looking and mapping relationships for the high cognitive group did not indicate that sequential looking was significantly associated with mapping behavior, however, discrepant sequential looking had the lowest insignificant value. Face variables revealed no significant associations with mapping variables. Like the typical cognitive group, eye gaze cues created differences in before and after mapping scores in the discrepant condition. For the follow-in condition, these mapping scores were associated with high values of insignificance. Correlations of eye gaze shift scores and mapping scores directly afterwards were insignificant. As with the entire study sample, intervention effects on before and after mapping scores only indicate that the show gesture intervention was significant, but the head turn intervention was not.
These looking and mapping relationships were also investigated for the diagnosis groups. Starting with the disability diagnosis group, discrepant sequential looking was again associated with mapping scores. Also a familiar counter theme, negative significant correlations were found for sequential looking during the follow-in condition. Face looking was not related to mapping scores. And as in the typical cognitive group, eye gaze cues were significantly related with mapping in the discrepant condition. Correlations of eye gaze scores and mapping scores directly afterwards were insignificant. Before and after mapping scores were significantly different for the show gesture intervention, but not the head turn intervention, as they were for the high cognitive group.
Analyses of looking and mapping scores for the autism diagnosis group were based on only four high functioning participants with autism. No significant correlations were found for any mapping variables and sequential looking, face looking, eye gaze cues, eye gaze scores, head turn interventions, or gesture interventions. Students with autism did not systematicly vary their mapping behavior in response to cues or interventions designed to facilitate looking at language targets.
ACUITY AND LANGUAGE MAPPING
General results of acuity and language mapping
As noted in the section on acuity and looking, interpretation of the analyses of acuity groups is confounded by the fact that most of the hyperopia group holds concurrent membership with the low cognitive group, most of the astigmatism group holds membership in the middle cognitive group, and most of the myopia group holds membership in the high cognitive group. Because of this lack of balance of acuity group membership in each cognitive group, comparisons of all acuity group performances cannot be made simultaneously. Instead, each refractive error acuity group will be compared to the normal acuity group within each cognitive group. The disability diagnosis groups also shares this same lack of balance, because it spans several cognitive groups. The autism diagnosis group has more balance, but members of this group generally have normal vision. The one participant that has autism and is a member of the myopia acuity group has a cognitive progression of 17.9 months, which is on the border of the middle and low cognitive groups. This participant also has no mapping data due to low warm-up scores.
Analyses of mapping data for the acuity groups began with the typical cognitive group. In that group, there were 21 members of the normal acuity group and 1 member of the hyperopia group who did not have mapping data due to low warm-up activity scores, so analyses could not be performed. Within the middle cognitive group, Mann-Whitney tests for differences in all mapping variables did not indicate any significant differences in mapping scores for the astigmatism group (n = 5) and the normal (n = 8) acuity groups. Within the high cognitive group, no significant differences were revealed for the myopia group (n = 2) and the normal group (n = 13) for any mapping variables. The hyperopia group within the low cognitive group did not have mapping data due to low warm-up scores, so analyses could not be completed.
Another attempt was made to find significance in mapping data for the acuity groups. With the assumption that power was lacking, the typical cognitive group and the middle cognitive comparison groups were combined and the analyses of mapping variables were repeated for the normal and astigmatism groups (the hyperopic child was excluded). Tests were significant for show find-it scores (p = .02) and total find it scores (p = .03). Scatterplots and bar graphs suggest that the astigmatism group outperformed the group with normal vision. (The typical cognitive group and the middle cognitive group do not differ on these measures. Values of significance are .30 or more on all values.)
In spite of possible cognitive confounds, absolute value of refractive error scores were correlated with mapping data within the combined middle and typical cognitive groups to determine if a relationship existed between mapping and degree of refractive error. It has already been shown that refractive error is associated with proportion of functioning with respect to chronological age (file indexes). Spearman rank correlations indicated significant relationships for head turn (rs = .77, p = .04) and show gesture mapping scores (rs = .84, p = .04), and show gesture find-it scores (rs = .71, p = .03). Higher values of refractive error were associated with higher mapping scores. Attempts to correlate absolute value of acuity and mapping variables for the entire study sample were unsuccessful. Similarly, correlations of file indexes and mapping variables for the entire sample were insignificant. For whatever reason, the absolute value of acuity was more highly associated with mapping variables than the age progressions or file indexes within the combined middle and typical cognitive groups.
Summary of Acuity and Mapping
For the analyses that could be performed, no differences in mapping variables were related to acuity groups within cognitive or diagnosis groups. This is not surprising, given the lack of power and many insignificant associations of language mapping variables with participant characteristics (cognitive and diagnosis groups).
CHAPTER 5
DISCUSSION
This chapter will follow a similar format as the previous chapter. The topics of discussion are: (1) language mapping of novel words, (2) looking in response to static eye gaze cues, eye gaze shift cues, head turn cues, and hand gesture cues, (3) acuity information, (4) relationships between acuity and looking, (5) relationships between looking and mapping, and (6) relationships between acuity and mapping.
FINDINGS
Language Mapping
Explaining Insignificant Findings
Mapping variables were not significantly related to participant characteristics. With rare exceptions, few of the many language mapping variables were statistically significantly different among groups. Few showed significant correlations with age progressions. Perhaps the lack of significance of the language mapping variables is simply indicative of the wide variation that occurs in language acquisition associated with normal development (see Chapter 2: Review of the Literature).
Another point to consider is that fast-mapping variables may measure expressive and receptive language in the process of acquisition. The documented wide range of language development rates have measured language that was already acquired. The lack of significance of participant characteristics and language mapping variables in this study may be due to both the individual differences in language learning rates and the individual differences in various learning strategies employed by the participants. Children may not only learn at different paces developmentally, they may also use short-term strategies of varying efficiency to learn novel labels.
Another conclusion that might be drawn is that the measures used for mapping proficiency were too imprecise to show variation among participants. However, warm-up scores, which were derived from similar procedures, were significantly associated with expressive age progressions, and were nearly significantly related with cognitive age progressions. Conversely, warm-up scores were measures of language which had already been acquired, and they would be expected to correlate with language tests contained in children's educational files.
Attenuation of the progressed variables may be responsible for these discrepancies between warm-up and language variable correlations. Warm-up scores and warm-up vocal scores were related to age progressed measures, while language mapping variables were unrelated to the progressions. Language mapping variables lacked the participants in the low cognitive group due to low warm-up scores, while the warm-up scores include the members of the low cognitive group. The absence of the lower functioning participants may have attenuated the progressed measures' contribution to correlations.
Lack of power which resulted from the exclusion of participants with low warm-up scores also may have skewed the language mapping data. The exclusion the low cognitive group due to low warm-up scores barred any interpretation of the mapping abilities of children who were functioning below the developmental age of 16.9 months. However, typical children of this age are not known for their abilities as fast-mappers, so these type of tests may not be valid for the low functioning participants. In addition, most of the low cognitive group was made up of children with autism, who are less likely to engage in language mapping behavior. The children in the typical and middle cognitive groups also suffered a loss of statistical power. Both groups lost four members due to low warm-up scores.
The explanations for the lack of significance are many, and range from population characteristics to measurement and statistical weaknesses. Perhaps a number of these explanations offered here are correct in some concurrent manner. Further revision of study design and participant sample is needed to improve conclusions about insignificance or improve the significance of the language variables themselves.
Significant Findings.
The rare significant findings were differences in the show gesture mapping and total find-it scores for participants in diagnosis groups. High functioning children with autism differed from their peers in the disability diagnosis group in terms of their total find-it scores and their ability to utilize show gestures to map novel words. Group medians show that the disabilities group outperformed the autism group and the no disability group. This would be expected behavior for children who are developmentally older (many participants in the disabilities group), and children with autism, who have difficulties with language. No differences were found for the typical cognitive group and the middle cognitive comparison group on any mapping variable.
Children who were savvy about mapping novel words in the naturalistic conditions (follow-in and discrepant) integrated vocalization in their responses in similar ways to their correct responses during the warm-up activity. Integration of gesture and speech during the warm-up activity was correlated with developmental levels. However, the vocal scores and vocal totals were not consistently greater for higher cognitive age progressions as expected. This difference may be due to individual variation in their style of expression upon learning novel words. More specific information on vocal and gestural integration in typical development may reveal differences when compared with nontypical development in this regard.
Baldwin (1993) found that roughly 75% of her 18-19 month old typical children demonstrated mapping in the discrepant condition. The typical children in the present study were older (17-32 months old), yet only 41 % of the children in this study chose the correct toy on the first verbal prompt in discrepant condition. Differences between these two studies could be explained by differences in the saliency of the researcher. Perhaps the children in Baldwin's study were closer to the researcher. In this study a card table separated the child and the researcher. The child was seated and the researcher was standing up. This might require the child to be more attentive to what the researcher was doing, in order to make language inferences based on researcher behavior.
It is also possible that the fast-mapping activities in this study were more challenging compared with Baldwin's study tasks. Most of the mapping variables were constructed around two consecutive prompts, which may have further increased task demands for young children. The youngest perfect mapper (+4 score for follow-in and discrepant conditions) in the typical group was 23.7 months. The youngest mapper in the disability groups had a cognitive age progression of 27.3 months. These children who succeeded in mapping demands of this study were older than the children who succeeded in the mapping demands of Baldwin's study.
LOOKING
Looking Variable Characteristics
In contrast with mapping variables, looking variables were frequently associated with age progressions, especially total sequential looking scores and total eye gaze shifting scores. Face looking scores were not significantly correlated with age progressed measures. This implies that the tendency to look at a communication partner's face in a naturalistic setting is not related to cognitive or language abilities, whereas sequential looking and eye gaze shifting are skills much more likely to be related to cognitive and language ability tests scores in children's file information.
Also, while the mapping variables showed few associations with participant characteristics, all types of looking variables showed consistent associations with participant characteristics. For instance, the cognitive groups differed on all types of looking variables and diagnosis groups demonstrated differences in sequential looking and eye gaze shifting scores.
The data also showed that sequential looking occurred more often during the discrepant condition than in the follow-in condition. A possible reason for this may be the increased effort required to refocus the eyes from a far target (e.g., the researcher's space) to a near target (e.g., the child's toy) in the follow-in condition. This refocusing is not as necessary in the discrepant condition. Furthermore, sequential looking in the follow-in condition requires the child to shift his or her eye gaze a greater number of degrees than the discrepant condition when matching the speaker's eye gaze. Perhaps typical children or children with disabilities are not as adept at refocusing while simultaneously shifting their gaze in large arcs.
Data on interventions designed to facilitate child looking at language targets revealed that show gesture interventions are more consistent than head turn interventions in obtaining improvements in looking for this study sample. Perhaps this is because a moving target attracts visual attention. Head turns may actually prevent some participants from looking at the intended language target because the head itself is moving. Show gestures, in comparison, move the intended language object, and may directly attract attention to the intended target. For children with autism, any movement that traced a significant arc in the their visual field seemed to be very useful in attracting visual attention. Perhaps the movement of the eye gaze shift cues was not salient enough for children with autism, because the arc traced by moving eyes was not great enough to attract attention.
It is also possible that the researcher's head turns were "too mechanical" to be effective. Perceptual motor research on gesture integration indicate that in natural head turns, the eyes meet the target first and the head turn aligns with the target last. The researcher's head turn, by comparison, may have turned the head and shifted the eyes simultaneously. Perhaps more effective head turning interventions would have followed the more naturalistic procedure.
Group Looking Characteristics
Most of the differences in looking behavior for cognitive groups were attributable to the differences between the high and low groups. Several looking variables were significantly related to cognitive progressions, so it is expected that groups based on this progression would demonstrate differences. The typical and middle cognitive comparison groups did not differ on looking variables, except for total sequential looking (discrepant sequential was almost significant). Also, the low cognitive group was the only group that might have looked more with head turn interventions than eye gaze scores. All groups looked at language targets more with show gestures than with head turns.
Additional evidence of the relative differences of sequential looking and face looking indicate that as children progress in development, there is a tendency to perform more sequential looking than face looking in both discrepant and follow-in conditions. The amount of face looking and sequential looking increased for the cognitive groups with higher developmental levels. However, the trend for sequential looking in the follow-in condition lags behind the trend for face looking. The middle group performed more face looking than discrepant looking in both the sequential and follow-in conditions. The typical comparison group performed like the middle group in the follow-in condition, but performed more sequential looking than face looking in the discrepant condition. The high cognitive group performed more sequential looking in both conditions. These descriptive data may be an indication of delayed neurological development of looking and/or focusing behavior for the middle group. Studies which include the measurement of sequential looking should take into account the condition imposed, the developmental level of the participant, and the degree of disability present. For this reason, conclusions based on the analyses of total scores should be made with caution.
Most of the differences between diagnosis groups on the sequential looking variables are attributable to the differences between the autism group and the disabilities group. Children in the autism diagnosis group did not perform sequential looking, face looking, or respond to eye gaze shifting cues. Their response to head turns often was to look at the turned head or to not look at all. Gesture interventions were the most successful at facilitating looking at language targets in children with autism.
Neurological development has often been suggested as a source of the lack of cognitive or language development in children with disabilities. Neurology may also affect the particular way that these children use their eyes. (This association was noted in Chapter 2: Review of the Literature.) Furthermore, neurological status may directly or indirectly affect the use of the eyes. Although it is not possible at this time to assign some types of looking to "involuntary" or "voluntary" functions, the following serves as an illustration of the previous suggestion. If eye gaze shifting to match a speaker's visual target is an involuntary function, neurological impairments could directly affect this process. On the other hand, if sequential looking is a voluntary function, indirect effects may be experienced if the child has no perceived need to perform that type of looking.
Children with autism perform very few of the looking behaviors associated with language learning, but it is not known at this time if their lack of language learning is caused by a neurological lack of cognition, or a neurological lack of looking behavior. The research and this data support both interpretations; most of the participants in this study who had autism were cognitively low functioning and did not perform looking behaviors associated with language abilities. The high functioning children with autism in this study did not perform these looking behaviors either.
Visual functioning of the children with autism in this study seemed qualitatively different than that of the other disabled or typical children. Members of the autism diagnosis group often squinted as if bright lights were being shined into their eyes when objects intersected large arcs of their visual field. Children with autism often would not respond to the sight of objects by reaching for them, but if objects were placed in their hands, they would respond to the objects with both tactile and visual interest.
ACUITY
Most of the participants with visual acuity problems were children with disabilities; they were members of the disabilities diagnosis group or the high, middle, and low cognitive groups. This information echoes that described in Chapter 2: Review of the Literature in the section on visual acuity in disability populations. It has been documented that people from disability populations are more likely to have vision problems, including less serious acuity problems. Theories about this connection have implicated flaws in neurological development and/or mishaps during the prenatal or perinatal periods. It was postulated that whatever had slowed cognitive or neurological development, had also prevented visual development.
Significant relationships were found between all developmental indexes (cognitive, expressive language, & receptive language) and the absolute value of refractive errors when the autism group was removed from analyses. High amounts of refractive error were associated with lower developmental indexes. The same analyses which included participants with autism still revealed significant relationships with two of the developmental indexes. This demonstrates a more direct relationship of the above theory. The lower the proportion of development in respect to chronological age, the greater the degree of visual impairment.
The above mentioned theory about mental and visual impairments in developmentally impaired populations does not adequately explain the case of autism, which is often associated with extreme developmental delays. In this study sample, typical participants and participants with autism had similarly low incidences of visual acuity problems. If there is a close association between the development of the eye and the development of the brain at the time of birth, why did the children with autism have normal vision? Is it possible that their impairment developed after the time of birth when the eyes were more fully developed? If autism has genetic causes, are the effects produced after the time of birth? Or, are the effects for another area of the brain that controls response to visual stimuli? Causes of autism cannot be debated here, but this is offered as another piece to that interminable puzzle.
All of the participants with identified ocular coordination problems were also members of disability groups; they were members of the disabilities and autism diagnosis groups, and also members of the high, middle, and low cognitive groups. Five of the eight participants with ocular coordination problems also had hyperopia or astigmatism. In Chapter 2 it was also noted that ocular coordination problems (strabismus) were prevalent in disability populations. This information is replicated in this study.
ACUITY AND LOOKING
Explaining Findings
Analyses of the relationships between visual acuity and looking behavior were difficult because of the lack of power and the confounds with developmental levels. Each acuity group had predominant membership within one of the cognitive groups. Therefore analyses were restricted to comparisons of each acuity group to the participants with normal vision in each cognitive group. Comparisons with diagnosis groups were not possible because the same cognitive confound applied for the disability and autism diagnosis groups (these groups spanned several cognitive groups).
The only significant differences for acuity group looking scores were found within the middle cognitive group, in which the astigmatism and normal acuity groups obtained significant differences on sequential looking scores during the discrepant condition. Means and scatterplots suggest that the sequential looking scores were actually higher for the astigmatism group than the normal acuity group. This implication is not easily explained: it could be said that this represented a compensation strategy for poor vision, or that looking was attributable to individual differences.
The lack of significant differences in looking scores for the low cognitive group may actually indicate that hyperopic participants have similar looking behavior to the autistic participants with normal vision. This suggestion is somewhat confounded by cognitive ages; low developmental levels may be responsible for the lack of looking behavior in autistic and developmentally delayed hyperopic participants. However, it is possible that hyperopia has affected the looking behavior of these participants, so that they are indistinguishable from their autistic peers with these measures. Baldwin's (1993) study demonstrated that typical children of 14-19 months all display similar looking behavior. This is not the case in this study, where differences are found for a number of looking variables between a number of groups. Also, children with disabilities, functioning developmentally from 10-15 months of age, did not perform similar looking behaviors as the rest of the study sample. Conversely, it is pointed out that these younger (estimated) developmental ages may not display similar looking patterns to children over 12 months of age. A typical and developmentally younger sample may produce less frequent looking behavior. However, this is not likely given the social savvy of preverbal children.
Ocular Coordination Concerns
Participants with ocular coordination problems were distinguished by difficulties in matching the speaker's direction of gaze (inaccurate following in response to eye gaze shifting cues) and inaccurate mapping to the location in space where they had previously inaccurately followed. Although participants from many different diagnosis, cognitive, or acuity groups performed inaccurate following behavior, the mere eight (8) participants that had binocular concerns had higher averages of inaccurate following than the 52 participants that had no documented binocular concerns. These findings are even more pronounced when considering that the mode frequency of inaccurate following for the "no concerns" group was zero (0) and the mode frequency for the "binocular concerns" group was six (6).
In addition to tracking problems, participants with binocular concerns were more likely to inaccurately map the novel label to the inaccurate location which the participants had chosen through inaccurate following behavior. Why is it that children without binocular concerns who performed inaccurate following did not also inaccurately map novel words to erroneous locations? Perhaps typical development includes some cognitive mechanism by which children are somehow aware that they did not accurately assess the gaze of the speaker. Children who are accurate gaze followers most of the time might be able to discern when they did not do so accurately. This mechanism may be intact in typical children and in some children with disabilities. In other children with disabilities the mechanism may not be intact due to lack of binocular coordination. If these children inaccurately follow the gaze of a speaker more of the time, they may loose (or never develop) the discernment of when they are inaccurate in following a speaker's gaze. As a result, they may perform more inaccurate mapping.
Additional tests were conducted in attempt to explain these results with other subject characteristics. No cognitive or diagnosis groups differed on inaccurate following or inaccurate mapping measures. Also, analyses were made of the data for only those participants that performed inaccurate following; no significant differences were found when comparing any cognitive or diagnosis groups on measures of inaccurate following or inaccurate mapping.
If binocular tracking problems are the cause of inaccurate following and inaccurate mapping, it is possible that children with these concerns are hindered by them in language acquisition. Inaccurate looking in response to speaker's eye gaze shifts may give rise to inaccurate mapping, and consequently children may also take longer to sort out the ambiguities that arise from conflicts caused from inaccurate assumptions. Teachers and other adults in the lives of children with disabilities may naturally take for granted the shared joint attention of children that have binocular issues. Children may miss many temporally oriented cues, such as shifting eye gaze or head turns, which often aid children in the acquisition of language. When a speaker's eyes are on a language target, it would be easy for a speaker not to notice that a child's eyes were not visually attentive to the same object. Perhaps children with disabilities may be more likely to map their inaccurate following on to other objects than those intended by the speaker.
It might be suggested that prism prescription glasses would cure the problem of inaccurate following (for children with strabismus). However, two of the eight children with binocular tracking concerns performed inaccurate following behavior in spite of prismatic glasses. It could be argued that perhaps these children had not learned to use these glasses correctly. However, of the five children in this study who wore glasses, four of them performed inaccurate following behavior even though there were no reported signs of ocular coordination problems. Perhaps an unintended side effect for children with disabilities who wear glasses is to actually produce inaccurate following. Alternatively, children with disabilities may not communicate precisely with their opticians, and end up wearing glasses that have an inaccurate prescription.
LOOKING AND LANGUAGE MAPPING
Explaining Looking & Mapping Relationships
For the entire study sample, the relationship between looking and mapping behavior is most consistently demonstrated with sequential looking variables, especially in the discrepant condition. The propensity to engage in this behavior, specifically in the discrepant condition, seems to be an indicator of general mapping ability. The fact that sequential looking did not occur equally in all conditions may be an indication that participants used other strategies to infer the speaker's referent (e.g., inferring attention to the child's toy). It may also be that children did not need to perform sequential looking as often as they did, but it was easier to do so in the discrepant condition. Because of the accumulation of evidence (see pgs. 50, 52, 62, 63, 64, 73, & 74) for the distinctness of sequential looking behavior in the follow-in and discrepant conditions, it is recommended that researchers be conscious of design conditions, developmental level and the presence of disability in the design of their research and data analyses.
In Baldwin's (1993) study typical children aged 14-19 months produced similar looking behavior, but only the children 18-19 months old used information gained from looking to map novel words to novel toys in both the discrepant and follow-in conditions. However, in this study sequential looking scores were related to cognitive age progressions. Children with disabilities with lower developmental ages performed less sequential looking than those who had older developmental ages. This relationship suggests that children with disabilities who purposely (and frequently) employ sequential looking strategies are higher functioning in addition to being better at language learning. Conversely, the relationship may be somewhat artificial due to the low functioning children with autism, whose lack of social referencing would strengthen this relationship.
For the entire study sample, eye gaze shift cues did not create differences in mapping scores directly before and after these cues. Also, eye gaze shift scores were not related to mapping scores directly afterward. This indicates that eye gaze shifting cues did not reliably help participants to map the novel words. Although some participants were able to utilize these cues to fast-map, others looked, but did not fast-map. Still others did not look or map when cues were given. Unlike sequential looking, which was related to both age progressed measures and language mapping, total eye gaze shift scores were related to age progressed measures, but not to language mapping. It is possible that participants had difficulties releasing their preconceived ideas about novel word meanings in favor of eye gaze shift cues in the naturalistic conditions. In the intervention conditions, where naturalistic conditions did not precede eye gaze shift cues, there may have been too few mentions of the novel word to learn it based on eye gaze shifting cue alone.
The study sample also demonstrated that show gesture interventions were more consistently associated with significant differences in mapping scores before and after the interventions. Head turn interventions were inconsistently significant, and in some conditions with some participants had negative effects (lower scores than eye gaze mapping scores). A general observation of all the looking variables in this study might be that children who manage their own visual attention through sequential looking are more likely to fast-map than children who have their visual attention managed for them through the use of eye gaze shift cues, head turns, or show gestures.
Explaining Study Group Profiles. Analyses of participant characteristics as they related to these looking and mapping relationships were conducted by examination of the individual cognitive and diagnosis groups. The typical cognitive group was much like the entire study sample in terms of its profile on looking and mapping variable relationships. Discrepant condition sequential looking was key to correlations with mapping variables. Eye gaze cues helped mapping scores in discrepant conditions, and significance was close in the follow-in condition. The middle cognitive group also revealed that discrepant sequential looking was an indicator of mapping variables. But surprisingly, follow-in sequential looking was negatively correlated with mapping scores, indicating that as follow-in sequential looking increased, mapping scores decreased. Peculiar to the middle group, face looking during follow-in condition was related to follow-in mapping scores (find-it game). Eye gaze cues did not create differences in mapping scores immediately before and after these cues.
Reasons for these differences between the typical and middle cognitive groups might be explained by neurological impairments, which might affect both cognitive and ocular-motor processes (motor movements required for eyes to move and focus). Face looking may be a "replacement" strategy for difficulties involved with sequential looking in the follow-in condition (e.g., difficulties with refocusing). This hypothesis is strengthened by the negative correlations of sequential looking and mapping in the middle cognitive group. Under these conditions, these participants may not have really performed a joint reference act (to the speaker and the object simultaneously), but instead they may have disengaged from the speaker entirely and returned to their own toy to resume play.
The differences between the typical and middle cognitive groups in terms of utilizing eye gaze shift cues are also intriguing. The typical group was able, at least in the discrepant condition (almost significantly in the follow-in condition), to correct errors in fast-mapping using eye gaze shift cues. The middle group was not able to use these cues in either condition, and significance values were quite high. Perhaps participants in the middle group were not cognitively flexible enough to let go of their preconceived notions about the meaning of novel words. This suggestion is in keeping with what is known about the longer processing time required by children with disabilities.
Looking and mapping variable relationships were also investigated for the diagnosis groups. For the disability diagnosis group, these relationships were much like the typical group. Discrepant sequential looking was again associated with mapping scores and negative significant correlations were found for sequential looking during the follow-in condition. For this group of participants with disabilities, face looking was not related to mapping scores in either naturalistic condition. As in the typical cognitive group, eye gaze cues were significantly related with mapping in the discrepant condition.
Analyses of looking and mapping scores for the autism diagnosis group were based on only four high functioning participants with autism. No significant correlations were found for any mapping variables and sequential looking, face looking, eye gaze cues, eye gaze scores, head turn interventions, or gesture interventions. Students with autism did not respond in systematic ways to opportunities to look or to cues or interventions designed to facilitate looking at language targets. The social referencing differences between children with autism and children with other disabilities has already been noted in the research literature.
ACUITY AND LANGUAGE MAPPING
As noted in the section on acuity and looking, interpretation of the analysis of acuity groups is confounded by the fact that most of the hyperopia group holds concurrent membership with the low cognitive group, most of the astigmatism group holds membership in the middle cognitive group, and the myopia group is mostly in the high cognitive group. Because of this lack of balance of acuity group membership in each cognitive group, comparisons of all acuity group performances on relationship between acuity and language mapping cannot be made simultaneously.
Therefore, each refractive error acuity group was compared to the normal acuity group within each cognitive group. The disability diagnosis group also shared the same lack of balance as the cognitive groups, because of the span in cognitive age progressions. The members in the autism diagnosis group had only one member with severe myopia, who did not have mapping data due to low warm-up scores.
Within the typical cognitive group, there were 21 members of the normal acuity group and one (1) member of the hyperopia group who did not have mapping data due to low warm-up scores, so analyses could not be performed. Within the middle cognitive group, no significant differences in mapping variables were found for the astigmatism (n = 5) and the normal (n = 8) acuity groups. Within the high cognitive group, no significant differences were revealed for the myopia (n = 2) and normal (n = 13) acuity groups for any mapping variables. Analyses of the low cognitive group could not be performed due to low warm-up scores.
The lack of significance described here is reminiscent of other analyses of the language variables. No participant characteristics are consistently associated with language mapping scores. The strongest connections with language scores were looking variables, especially sequential looking. If sequential looking groups had been formed, it is likely that differences would have emerged for language variables. This also concurs with previous research which has documented relationships between joint attention and expressive vocabulary or future language development (see Chapter 2). The protocol used in this study might be further refined to develop "looking groups" which more accurately explain differences in language development.
LIMITATIONS
All research activities of this project were carried out by the author. This may have created a bias in the collection of data, coding of videotapes, and interpretation of statistical analyses. However, some circumstances of this project make the possibility of bias less likely. (1) The protocol used during data collection was mentally demanding. This and the need to keep participant interest high may have prevented the researcher from conscious manipulation of data. (2) The coding of videotapes was straightforward. Responses to looking targets and mapping opportunities were clearly defined before data collection. (3) The interpretation of statistical analyses were made in a variety of ways to ensure that findings were accurate. The findings that were the strongest had the convergence of several different tests. (4) The main findings concerning language mapping and visual acuity were inconclusive. Few findings concerning the original questions about a possible relationship between visual acuity and language mapping were found. No cognitive or diagnosis group demonstrated language differences.
Durning data collection, the lack of affirmative feedback seemed to confuse some of the more savvy participants in the high cognitive group. These participants would sometimes choose the correct object after naturalistic conditions (follow-in or discrepant), but then switch to the other incorrect object on the second prompt because the researcher did not affirm their first choice vigorously enough. Participants may have interpreted prompts to repeat their responses as gentle indications of their incorrectness. Adjustment in protocol to affirm all choices may also similarly backfire, because participants may be further entrenched in wrong choices, and be rendered impervious to eye gaze or gesture interventions. More pilot studies need to be conducted in order to improve the protocol in this regard.
Some very savvy participants were not sufficiently amused to show the researcher what they knew about novel words and created their own game dubbed by this researcher as the "not game." After a few successful trials, participants would purposefully choose the wrong object or purposely look the opposite way of eye gaze or head turn cues. Measures could be developed to exclude "not game players." Vocally integrated responses may decrease during purposeful incorrect choices, when participants are cognitively preoccupied with their mischievous fun.
The protocol specified that participants which were successful at different points in the protocol did not receive further interventions. This had the effect of decreasing statistical power for eye gaze shifting interventions and for many parts of Phase Two interventions. More statistically significant relationships might have been found if the design of this study had given the same prompts to all participants. However, this presents the problem of redundancy and boredom for savvy children, and may increase incidences of the "not" game described above. Also, low functioning children with autism were not able to tolerate long periods of time where they were not allowed to tactilely engage novel objects (e.g., during mapping prompts). Since the mapping data of participants with low warm-up scores cannot be used, mapping prompts could be left out of the protocol for these participants. Shortened protocols would solve this problem for low-functioning participants with autism.
Acuity groups lacked statistical power. Larger sample sizes are needed in order to identify enough participants with acuity problems that do not already have corrective prescriptions. Specific studies might also be conducted on populations that are likely to yield a specific refractive error (e.g. low functioning children without autism may have higher incidences of hyperopia).
Furthermore, the interest level that the novel toys produce in children may also play a part in language mapping. The pilot test of this study revealed that the first four toys were too interesting, which caused sequential looking to be less frequent than in the present study.
FUTURE RESEARCH
Aside from the research suggestions listed in the previous section on study limitations, future research could further investigate the areas of strength highlighted in this study. For instance, this study sample demonstrated (1) relationships between sequential looking or eye gaze shifting responses and language mapping variables, (2) relationships between sequential looking or eye gaze shifting responses and developmental levels, (3) relationships between use of show gestures and increased looking or mapping behavior, (4) relationships between acuity and developmental indexes (proportion of development with respect to chronological age), and (5) relationships between ocular coordination concerns and inaccurate following or inaccurate mapping.
1. This study could be augmented by research which determines whether sequential looking and eye gaze shifting behavior is the same for typical and disabled participants, and at what developmental levels they vary, if at all. Sequential looking may be more difficult for children with disabilities, even if they are functioning at the same developmental level as typical children. Is it more difficult for typical children and children with disabilities to shift focus from far to near targets? Face looking behavior may be a "substitute" for difficult sequential looking in follow-in conditions like that used in this study. Furthermore, relationships of looking behaviors and mapping scores could be replicated.
2. This study demonstrated that sequential looking and eye gaze shifting behaviors were related to age progressed measures. If sequential looking is related to language development in some way, it might be possible to develop a screening instrument based on these measures that would identify children with possible language problems (similar to joint attention tests for autism). Language Delay is the most frequent reason for referral to ECSE programs. The assessments that must be administered are expensive. Currently, these tests are based on chronological age, which is documented (by some of these same tests) to embrace a wide range of acquisition rates. If sequential looking, face looking, and eye gaze shifting behaviors are more defined for populations with and without disabilities, it may be possible to create an accurate and sensitive test for screening purposes.
3. It was easier to cause participants in this study to look at a language object than to actually map a novel word on to that object. Brief interventions were not fully successful in teaching children new words. It is possible that show gesture or head turn interventions would be more effective if the novel word was repeated more times. Perhaps modifications would help, such as ending the gesture with the object in the child's hands (e.g. "give" gesture). Tactile cues may be more effective than visual cues for teaching mapping to higher functioning children with autism or even to typical children. Other gestures could also be utilized for teaching language to children with and without disabilities.
4. A relationship between developmental indexes and visual acuity was noted in this study. Excluding children with autism, what is the incidence of normal acuity in the severe ranges of disability, where children are functioning at less than 25% of their chronological ages? How many of these disabilities were acquired in the prenatal or perinatal periods? How many were acquired after normal development had occurred? Answers to these questions may further solidify theories that were purported several decades ago by researchers of visual development.
5. Participants with ocular coordination problems demonstrated inaccurate following and inaccurate mapping behavior. Will this association be affirmed in other studies that specifically design their samples to study this question? Do prismatic glasses help these children to follow eye gaze cues more accurately? Do any kind of glasses increase the likelihood that children will follow eye gaze inaccurately? What interventions (e.g., gestures) will aid children with binocular coordination problems to follow eye gaze shifts more accurately? If children inaccurately map as a result of inaccurate following, how flexible are these children in abdicating incorrect assumptions about language targets?
Other questions that might be answered by future research include the following: If typical and cognitively matched children with moderate-severe disabilities are led to believe that a certain object is called by a novel name, how easily will they give up that understanding in favor of a contradictory cue? What types of cues are successful in getting children to change their minds? What types of previous experience prevent children from changing their minds? If typical children are more easily led to change their minds than children with disabilities, this may strengthen the suggestion that typical children are more flexible in their language mapping abilities, because they were able to utilize eye gaze cues, whereas children with disabilities were not.
Although there are many tangents that could be pursued from this study, the most useful to children with disabilities would be those questions central to normal development. Ultimately, children with disabilities are the ones the most in need of information on interventions that may help them to learn language. It would be useful to know what strategies they do and do not use when learning new words. However, more information on normal development and use of looking strategies is essential if comparisons are to be made with children who have disabilities.
REFERENCES
Acredolo, L., & Goodwyn, S. (1988). Symbolic gesturing in normal infants. Child Development, 59 (2), 450-466.
Allen, R., & Shatz, M. (1983). "What says meow?" The role of context and linguistic experience in very young children's responses to what-questions. Journal of Child Language, Vol 10 (2), 321-335.
Ameder, L., Peck, L., & Howland, H. (1990). Prevalence of anisometropia in volunteer laboratory and school screening populations. Investigative Opthalmology & Visual Science, 31 (11), 2448-2455.
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders (4th ed.). Wahington, D.C.: Author.
Bakeman, R., & Adamson, L. (1984). Coordinating attention to people and objects in mother-infant and peer-infant interactions. Child Development, 55 (4), 1278-1289.
Bakeman, R., & Adamson, L. (1986). Infants' conventionalized acts: Gestures and words with mothers and peers. Infant Behavior & Development, 9 (2), 215-230.
Baldwin, D. (1993). Infants' ability to consult the speaker for clues to word reference. Journal of Child Language, 20, 395-418.
Baron-Cohen, S., Baldwin, D., Crowson, M. (1997). Do children with autism use the speaker's direction of gaze strategy to crack the code of language? Child Development, 68 (1), 48-57.
Bates, E. (1979). Intentions, conventions, and symbols. In E. Bates, L. Benigni, I. Bretherton, L. Camaioni, & V. Volterra (eds.), The Emergence of Symbols: Cognition and Communication in Infancy, pp. 33-68. N.Y., N.Y.: Academic Press.
Bates, E., Benigni, L., Bretherton, I., Camaioni, L., & Volterra, V. (1979). Cognition and communication from nine to thirteen conths: Correlational findings. In E. Bates, L. Benigni, I. Bretherton, L. Camaioni, & V. Volterra (eds.), The Emergence of Symbols: Cognition and Communication in Infancy, pp. 69-140. N.Y., N.Y.: Academic Press.
Bates, E., Marchman, V., Thal, D., Fenson, L., Dale, P., Reznick, J., Reilly, J., & Hartung, J. (1994). Developmental and stylistic variation in the composition of early vocabulary. Journal of Child Language, 21 (1), 85-123.
Bebko, J. (1990). Echolalia, mitigation, and autism: Indicators from child characteristics for the use of sign language and other augmentative language systems. Sign Language Studies, 66, 61-78.
Benedict, H. (1979). Early lexical development: Comprehension and production. Journal of Child Language, 6, 183-200.
Blount, R., Baer, R., & Collins, F. (1984). Improving visual acuity in a myopic child: Assessing compliance and effectiveness. Behaviour Research & Therapy, 22 (1), 53-57.
Bourgeois, M. (1971). Olfactory hallucinations and parosmic anxiety. Annales Medico-Psychologiques, 1 (3), 377-402.
Butler, K. (1995). Learners with language impairments. In M. Wang, M. Reynolds, & H. Walberg (eds.), Handbook of Special and Remedial Education: Research and Practice, 2nd edition, pp. 303-340. N.Y., N.Y.: Elsevier Science, Inc.
Capirci, O., Iverson, J. Pizzuto, E., & Volterra, V. (1996). Gestures and words during the transition to two-word speech. Journal of Child Language, 23 (3), 645-673.
Caputo, A., Wagner, R., Reynolds, D., Guo, S., & Goel A. (1989). Down syndrome: Clinical review of ocular features. Clinical Pediatrics, 28 (8), 355-358.
Cardoso-Martins, C., & Mervis, C. (1990). Mothers' use of substantive deixis and nouns with their children with Down syndrome: Some discrepant findings. American Journal on Mental Retardation, 94 (6), 633-637.
Carter, D. (1964). Effects of prolonged wearing of prism. American Journal of Optometry & Archives of the American Academy of Optometry, 40 (4), 265-273.
Caselli, M. (1983). Communication to language: deaf children's and hearing children's developoment compared. Sign Lanugage Studies, 39, 113-144.
Caselli, M., Bates, E., Casadio, P., Fenson, L., Sanderl, L., & Weir, J. (1995). A cross-linguistic study of early lexical development. Cognitive Development, 10 (2), 159-199.
Caselli, M., Vicari, S., Longobardi, E., Lami, L., Pizzoli, C., Giacomo, S. (1998). Gestures and words in early development of children with Down syndrome. Journal of Speech, Language, & Hearing Research, 41 (5), 1125-1135.
Castane, M., Peris, E., & Sanchez, E. (1995). Ocular dysfunction associated with mental handicap. Ophthalmic & Physiological Optics, 15 (5), 489-492.
Clark, E. & Sengul, C. (1978). Strategies in the acquisition of deixis. Journal of Child Language, 5 (3), 457-475.
Cohen, D., Volkmar, F., & Paul, R. (1986). Issues in the classification of pervasive developmental disorders: History and current status of nosology. Journal of the American Academy of Child and Adolescent Psychiatry, 25 (2), 158-161.
Courtney, G. (1971). Refractive errors in institutionalized mentally retarded and emotionally disturbed children. American Journal of Optometry & Archives of the American Academy of Optometry, 48 (6), 492-496.
Dijkxhoorn, Y., Berckelaer-Onnes, I., & Ploeg, D. (1996). Enhancing communication in early childhood. In M. Brambring, H Rauh, & A. Beelmann (eds.), Early Childhood Intervention, pp. 403-418. New York, N.Y.: Walter de Gruyter.
Dobson, V., Fulton, A., Manning, K., Salem, D., & Petersen, R. (1981). Cycloplegic refractions of premature infants. American Journal of Opthalmology, 91 (4), 490-495.
Dote-Kwan, J., & Chen, D. (1995). Learners with visual impairments. In M. Wang, M. Reynolds, & H. Walberg (eds.), Handbook of Special and Remedial Education: Research and Practice, 2nd edition, 205-228. N.Y., N.Y.: Elsevier Science, Inc.
Dunlea, A. (1989). Vision and the Emergence of Meaning: Blind and Sighted Children's Early Language, pp. 35-67. N.Y., N.Y.: Cambridge University Press.
Evans, M., & Rubin, K. (1979). Hand gestures as a communicative mode in school-aged children. Journal of Genetic Psychology, 135 (2), 189-196.
Ferrell, K., & Raver, S. (1991). Social and communication development in infancy. In S. Raver (ed.), Strategies for Teaching At-Risk and Handicapped Infants and Toddlers: A Transdisciplinary Approach, 105-136. N.Y., N.Y.: Macmillan Publishing Co.
Flax, N. (1993). The treatment of strabismus in the four to ten year old child. Child & Adolescent Social Work Journal, 10 (5), 411-416.
Fraiberg, S. (1977). The sign system. In S. Fraiberg (ed.), Insights from the Blind: Comparative Studies of Blind and Sighted Infants, pp. 92-112. N.Y., N.Y.: Basic Books, Inc.
Franco, F., & Butterworth, G. (1996). Pointing and social awareness: Declaring and requesting in the second year. Journal of Child Language 23 (2), 307-336.
Franco, F., & Wishart, J. (1995). Use of pointing and other gestures by young children with Down syndrome. American Journal on Mental Retardation, 100 (2),160-182.
Getman, G. (1985). A commentary on vision training. Journal of Learning Disabilities, 18 (9), 505-512.
Glenn, S. & Cunningham, C. (1984). Nursery rhymes and early language acquisition by mentally handicapped children. Exceptional Children, 51 (1), 72-74.
Goldfield, B. (1985-86). Referential and expressive language: A study of two mother-child dyads. First Language, 6 (17, Pt 2), 119-131.
Goldin-Meadow, S., & Morford, M. (1985). Gesture in early child language: Studies of deaf and hearing children. Merrill-Palmer Quarterly, 31 (2), 145-176.
Goldin-Meadow, S., Seligman, M., & Gelman, R. (1976). Language in the two-year-old. Cognition, 4 (2), 189-202.
Goodhart, F., Baron-Cohen, S. (1993). How many ways can the point be made? Evidence from children with and without autism. First Language, 13 (38, Pt 2), 225-233.
Goodwyn, S., Acredolo, L. (1993). Symbolic gesture versus word: Is there a modality advantage for onset of symbol use? Child Development, 64 (3), 688-701.
Gopnik, A. (1988). Three types of early words: The emergence of social words, names and cognitive-relational words in the one-word stage and their relation to cognitive development. First Language, 8 (22, Pt 1), 49-69.
Greenspan, S., & Wieder, S. (1998). The Child with Special Needs: Encouraging Intellectual and Emotional Growth. Reading, MA: Addison-Wesley.
Grisham, J., & Simons, H. (1986). Refractive error and the reading process: A literature analysis. Journal of the American Optometric Association, 57 (1), 44-55.
Grosvenor, T. (1970). Refractive state, intelligence test scores, and academic ability. American Journal of Optometry & Archives of the American Academy of Optometry, 47 (5), 355-361.
Grosvenor, T. (1971). The neglected hyperope. American Journal of Optometry & Archives of the American Academy of Optometry, 48 (5), 376-382.
Harris, S., & Weiss, M. (1998). Topics in Autism: Right from the start. Behavioral Intervention for Young Children with Autism: A Guide for Parents & Professionals. Bethesda, MD: Woodbine Publishers.
Haslett, B. & Samter, W. (1997). Children Communicating: The first five years. Mahwah, N.J.: Lawrence Erlbaum Associates, Inc., pp. 20-56.
Hatton, D., Bailey, D., Burchinal, M., & Ferrell, K. (1997). Developmental growth curves of preschool children with vision impairments. Child Development, 68 (5), 788-806.
Herman, J., Tauber, E., & Roffwarg, H. (1974). Monocular occlusion impairs stereoscopic acuity, but total visual deprivation does not. Perception & Psychophysics, 16 (2), 225-228.
Hewitt, L. (1998). A social interactionist view of autism and its clinical management. Journal of Communication Disorders, 31 (2), 87-92.
Hirsch (1951-52). Visual anomalies among children of grammar school age. Journal of the American Optometric Association, 23 (11), 663-671.
Hirsch, M. (1964). Predictability of refraction at age 14 on the basis of testing at age 6: Interim report from the Ojai longitudinal study of refraction. American Journal of Optometry & Archives of the American Academy of Optometry, 41 (10), 567-573.
Hirsch, M., & Nadell, M. (1958). The relationship between intelligence and the refractive state in a selected high school sample. American Journal of Optometry & Archives of the American Academy of Optometry, 35 (6), 321-326.
Hobson, R., Lee, A. (1998). Hello and goodbye: A study of social engagement in autism. Journal of Autism & Developmental Disorders, 28 (2), 117-127.
Howland, H. (1985). Optics of photoretinoscopy: Results from ray tracing. American Journal of Optometry & Physiological Optics, 62 (9), 621-625.
Howland, H. (1988). Visual development: Optical aspects of the eye. In E. Meisami and P. Timiras (eds.), Handbook of Human Growth and Developmental Biology, pp. 155-163. Boca Raton, FL: CRC Press.
Howland, H. (1991). Refractive Errors: Review of the literature. Manuscript submitted for publication. Cornell University, Ithaca, N.Y.
Hyvahyvarinen, L. (1991). LH Symbols: Measurement Visual Acutity for Children. Long Island City, N.Y.: Lighthouse Low Vision Products.
Iverson, J., Capirci, O., & Caselli, M. (1994). From communication to language in two modalities. Cognitive Development, 9 (1), 23-43.
Jackson, P., & Vessey, J. (1996). Primary Care of the Child with a Chronic Condition, 2nd edition. St. Louis, MO: Mosby Publishers.
Janicki, M., Lubin, R., & Friedman, E. (1983). Variations in characteristics and service needs of persons with autism. Journal of Autism & Developmental Disorders, 13 (1), 73-85.
Jeffree, D., Wheldall, K., & Mittler, P. (1973). Facilitating two-word utterances in two Down's syndrome boys. American Journal of Mental Deficiency, 78 (2), 117-122.
Kaiser, A. (1993). Functional language. In M. Snell (ed.), Instruction of Students with Severe Disabilities, pp. 347-379. N.Y., N.Y.: Macmillan Publishing Co.
Kaplan, M., Carmody, D., Gaydos, A. (1996). Postural orientation modifications in autism in response to ambient lenses. Child Psychiatry & Human Development, 27 (2), 81-91.
Kasari, C., Freeman, S., Mundy, P., & Sigman, M. (1995). Attention regulation by children with Down syndrome: Coordinated joint attention and social referencing looks. American Journal on Mental Retardation, 100 (2), 128-136.
Kasari, C., Mundy, P., Yirmiya, N., & Sigman, M. (1990). Affect and attention in children with Down syndrome. American Journal on Mental Retardation, 95 (1), 55-67.
Kasari, C., Sigman, M., Mundy, P., & Yirmiya, N. (1990). Affective sharing in the context of joint attention interactions of normal, autistic, and mentally retarded children. Journal of Autism and Developmental Disorders, 20 (1), 87-100.
Kirschen, M. (1954). A study of visual performance of mentally retarded children. American Journal of Optometry & Archives of the American Academy of Optometry, 41(6), 282-288.
Koegel, R., Koegel, L., Surratt, A. (1992). Language intervention and disruptive behavior in preschool children with autism. Journal of Autism & Developmental Disorders, 22 (2), 141-153.
Koegel, R., O'Dell, M., & Koegel, L. (1987). A natural language teaching paradigm for nonverbal autistic children. Journal of Autism & Developmental Disorders, 17 (2), 187-200.
Kohen-Raz, R., Volkmar, F., Cohen, D. (1992). Postural control in children with autism. Journal of Autism & Developmental Disorders, 22 (3), 419-432.
Kohl, P., & Samek, B. (1988). Refractive error and preferential looking visual acuity in infants 12-24 months of age: Year 2 of a longitudinal study. Journal of the American Optometric Association, 59 (9), 686-690.
Kolb (1962). Examining the mentally retarded child. American Journal of Optometry & Archives of the American Academy of Optometry, 39 (12), 669-679.
Lerner, J., Lowenthal, B., & Egan, R. (1998). Preschool Children with Special Needs, 204-228. Boston, MA: Allyn & Bacon.
Leung, J., Yap, M., & Lagrow, S. (1992). Behavioral vision training for hyperopia. Psychologia: An International Journal of Psychology in the Orient, 35 (4), 232-239.
Light, J., Roberts., B., Dimarco, R., & Greiner, N. (1998). Augmentative and alternative communication to support receptive and expressive communication for people with autism. Journal of Communication Disorders, 31 (2), 153-180.
Lovaas, I. (1997). Intensive behavioral treatment for preschoolers with severe mental retardation and pervasive developmental disorder. American Journal on Mental Retardation, 102 (3), 238-249.
Manley, J., & Schuldt, W. (1970). The refractive state of the eye and mental retardation. American Journal of Optometry & Archives of the American Academy of Optometry, 47(3), 236-241.
Matson, J., Benavidez, D., Compton, L., Paclawskyj, T., & Baglio, C. (1996). Behavioral treatment of autistic persons: A review of research from 1980 to the present. Research in Developmental Disabilities, 17 (6), 433-465.
McArthur, D., & Adamson, L. (1996). Joint attention in preverbal children: Autism and developmental language disorder. Journal of Autism & Developmental Disorders, 26 (5), 481-496.
McNeill, D. (1985). Hand and Mind, pp. 295-328. Chigaco, Ill.: The University of Chicago Press.
Mills, A. (1983). Acquisition of speech sounds in the visually handicapped child. In A. Mills (ed.), Language Acquisition in the Blind Child: Normal and Deficient, 46-56. SanDiego, CA: College-Hill Press, Inc.
Mohindra, I., Jacobson, S., Zwaan, J., & Held, R. (1983). Psychophysical assessment of visual acuity in infants with visual disorders. Behavioural Brain Research, 10 (1), 51-58.
Morales, M., Mundy, P., & Rojas, J. (1998). Following the direction of gaze and language development in 6-month-olds. Infant Behavior & Development, 21 (2), 373-377.
Morford, M., & Goldin-Meadow, S. (1992). Comprehension and production of gesture in combination with speech in one-word speakers. Journal of Child Language, 19 (3), 559-580.
Morrison, M., & Polloway, E. (1995). In E. Meyen & T. Skrtic (eds.), Mental retardation. Special Education & Student Disabilitiy, an Introduction: Traditional, Emerging, and Alternative Perspectives, pp. 213-269. Denver, CO: Love Publishing Co.
Mundy, P. (1995). Joint attention and social-emotional approach behavior in children with autism. Development & Psychopathology, 7 (1), 63-82.
Mundy, P., & Gomes, A. (1998). Individual differences in joint attention skill development in the second year. Infant Behavior & Development, 21 (3), 469-482.
Mundy, P., & Sigman, M. (1989). The theoretical implications of joint-attention deficits in autism. Development & Psychopathology, 1 (3), 173-183.
Mundy, P., Kasari, C., & Sigman, M. (1992). Nonverbal communication, affective sharing, and intersubjectivity. Infant Behavior & Development, 15 (3), 377-381.
Mundy, P., Kasari, C., Sigman, M., & Ruskin, E. (1995). Nonverbal communication and early language acquisition in children with Down syndrome and in normally developing children. Journal of Speech & Hearing Research, 38 (1), 157-167.
Mundy, P., Sigman, M., & Kasari, C. (1990). A longitudinal study of joint attention and language development in autistic children. Journal of Autism & Developmental Disorders, 20 (1), 115-128.
Mundy, P., Sigman, M., & Kasari, C. (1994). Joint attention, developmental level, and symptom presentation in autism. Development & Psychopathology, 6 (3), 389-401.
Mundy, P., Sigman, M., Kasari, C., & Yirmiya, N. (1988). Nonverbal communication skills in Down syndrome children. Child Development, 59 (1), 235-249.
Mundy, P., Sigman, M., Ungerer, J., & Sherman, T. (1986). Defining the social deficits of autism: The contribution of non-verbal communication measures. Journal of Child Psychology & Psychiatry & Allied Disciplines, 27 (5), 657-669.
Namy, L. & Waxman, S. (1998). Words and gestures: Infants' interpretations of different forms of symbolic reference. Child Development, 69 (2), 295-308.
Norcia, A., Hamer, R., Jampolsky, A., & Orel-Bixler, D. (1995). Plasticity of human motion processing mechanisms following surgery for infantile esotropia. Vision Research, 35 (23-24), 3279-3296.
Ottar, W., Scott, W., & Holgado, S. (1995). Photoscreening for amblyogenic factors. Journal of Pediatric Ophthalmology and Strabismus, 32 (5), 289-295.
Pechmann, T., & Deutsch, W. (1982). The development of verbal and nonverbal devices for reference. Journal of Experimental Child Psychology, 34 (2), 330-341.
Peterson, S., Bondy, A., Vincent, Y., Finnegan, C. (1995). Effects of altering communicative input for students with autism and no speech: Two case studies. Augmentative and Alternative Communication, 11 (2), 93-100.
Pierce, K., Glad, K., & Schreibman, L. (1997). Social perception in children with autism: An attentional deficit? Journal of Autism and Developmental Disorders, 27 (3), 265-282.
Reich, R. (1978). Gestural facilitation of expressive language in moderately/severely retarded preschoolers. Mental Retardation, 16 (2), 113-117.
Rescorla, L. (1980). Overextension in early language development. Journal of Child Language, 7 (2), 321-335.
Rescorla, L. (1981). Category development in early language. Journal of Child Language, 8 (2), 225-238.
Rice, M., & Schuele, C. (1995). Speech and language impairments. In E. Meyen & T. Skrtic (eds.), Mental retardation. Special Education & Student Disabilitiy, an Introduction: Traditional, Emerging, and Alternative Perspectives, pp. 339-374. Denver, CO: Love Publishing Co.
Roeyers, H., Van Oost, P., & Bothuyne, S. (1998). Immediate imitation and joint attention in young children with autism. Development & Psychopathology, 10 (3), 441-450.
Rogers-Warren, A., & Warren, S. (1984). The social basis of language and communication in severely handicapped preschoolers. Topics in Early Childhood Special Education, 4 (2), 57-72.
Rogoff (1990). Apprenticeship in Thinking, pp. 65-85. N.Y., N.Y.: Oxford University Press.
Rollins, R., Wambacq, I., Dowell, D., Mathews, L., & Reese, P. (1998). An intervention technique for children with autistic spectrum disorder: Joint attentional routines. Journal of Communication Disorders, 31 (2), 181-193.
Rondal, J. (1988). Down syndrome. In, D. Bishop, & K. Mogford (eds.), Language Development in Exceptional Circumstances, pp. 165-176. N.Y., N.Y.: Churchill Livingstone.
Rose, M., & Torgerson, N. (1994). A behavioral approach to vision and autism. Journal of Optometric Vision Development, 25 (4), 269-275.
Rosner, J., & Rosner, J. (1997). The relationship between moderate hyperopia and academic achievement: How much plus is enough? Journal of the American Optometric Association, 68 (10), 648-650.
Rossetti, L. (1991). Social and communication development in infancy. In S. Raver (ed.), Strategies for Teaching At-Risk and Handicapped Infants and Toddlers: A Transdisciplinary Approach, 105-136. N.Y., N.Y.: Macmillan Publishing Co.
Rowland, C. (1984). Preverbal communication of blind infants and their mothers. Journal of Visual Impairment and Blindness, Sept., 297-302.
Sachs, J., & Truswell, L. (1978). Comprehension of two-word instructions by children in the one-word stage. Journal of Child Language, 5 (1), 17-24.
Salmon, C., Rowan, L., & Mitchell, P. (1998). Facilitating prelinguistic communication: Impact of adult prompting. Infant-Toddler Intervention, 8 (1), 11-27.
Sameroff, A., & Chandler, M. (1975). Reproductive risk and the continuum of caretaking casualty. In F. Horowitz, E. Hetherington, S. Scarr-Salapatek, & G. Siegel (eds.), Review of Child Development Research, vol. 4, pp. 187-244. Chicago, Ill.: The University of Chicago Press.
Schieffelin, B., & Ochs, E. (1983). A cultural perspective on the transition from prelinguistic to linguistic communication. In R. Golinkoff (ed.), The Transition from Prelinguistic to Linguistic Communication, pp. 115-133. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers.
Schnur, E., & Shatz, M. (1984). The role of maternal gesturing in conversations with one-year-olds. Journal of Child Language, Vol 11 (1), 29-41.
Schulman, R. (1994). Optometry's role in the treatment of autism. Journal of Optometric Vision Development, 25 (4), 259-268.
Schwartz, R. (1938). Ocular factors in poor readers in the Saint Louis public schools. American Journal of Optometry & Archives of the American Academy of Optometry, 23 (5), 535-538.
Schwartz, I., Carta, J., & Grant, S. (1996). Examining the use of recommended language intervention practices in early childhood special education classrooms. Topics in Early Childhood Special Education, 16 (2), 251-272.
Seifer, R., Schiller, M., Sameroff, A., Resnick, S., & Riordan, K. (1996). Attachment, maternal sensitivity, and infant temperament during the first year of life. Developmental Psychology, 32 (1), 12-25.
Siegel-Causey, E., & Wetherby, A. (1993). Nonsymbolic communication. In M. Snell (ed.), Instruction of Students with Severe Disabilities, pp. 290-318. N.Y., N.Y.: Macmillan Publishing Co.
Slavik, B. (1982). Vestibular function in children with nonparalytic strabismus. Occupational Therapy Journal of Research, 2 (4), 220-233.
Slobin, D. (1972). Children and language: They learn the same way all around the world. Psychology Today, 6 (2), 71-74, 82.
Stewart-Brown, S., Haslum, M., & Butler, N. (1985). Educational attainment of 10-year-old children with treated and untreated visual defects. Developmental Medicine & Child Neurology, 27 (4), 504-513.
Sugarman, S. (1983). Discussion: Empirical versus logical issues in the transition from prelinguistic to linguistic communication. In R. Golinkoff (ed.), The Transition from Prelinguistic to Linguistic Communication, pp. 133-145. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers.
Sugarman, S. (1984). Why talk? Comment on Savage-Rumbaugh et al. Journal of Experimental Psychology, 112 (4), 493-497.
Tardif, T., Shatz, M., & Naigles, L. (1997). Caregiver speech and children's use of nouns versus verbs: A comparison of English, Italian, and Mandarin. Journal of Child Language, 24 (3), 535-565.
Teller, D., Morse, R., Borton, R., & Regal, D. (1974). Visual acuity for vertical and diagonal gratings in human infants. Vision Research, 14 (12), 1433-1439.
Thal, D., & Bates, E. (1988). Language and gesture in late talkers. Journal of Speech & Hearing Research, 31 (1), 115-123.
Thompson, L., Massaro, D. (1986). Evaluation and integration of speech and pointing gestures during referential understanding. Journal of Experimental Child Psychology, Vol 42 (1), 144-168.
Tomasello, M. & Todd, J. (1983). Joint attention and lexical acquisition style. First Language, 4 (12, Pt 3), 197-211.
Warren, S., Yoder, P., Gazdag, G., & Kim, K., & Jones, H. (1993). Facilitating prelinguistic communication skills in young children with developmental delay. Journal of Speech & Hearing Research, 36 (1), 83-97.
Waterhouse, L., Morris, R., Allen, D., Dunn, M., Fein, D., Feinstein, C., Rapin, I., & Wing, L. (1996). Diagnosis and classification in Autism. Journal of Autism and Developmental Disorders, 26 (1), 59-86.
Watson, L. (1998). Following the child's lead: Mother's interactions with children with autism. Journal of Autism and Developmental Disorders, 28 (1), 51-60.
Wetherby, A., & Prutting, C. (1984). Profiles of communicative and cognitive-social abilities in autistic children. Journal of Speech & Hearing Research, 27 (3), 364-377.
Wetherby, A., &Rodriguez, G. (1992). Measurement of communicative intentions in normally developing children during structured and unstructured contexts. Journal of Speech & Hearing Research, 35 (1), 130-138.
Wetherby, A., Cain, D., Yonclas, D., Walker, V. (1988). Analysis of intentional communication of normal children from the prelinguistic to the multiword stage. Journal of Speech & Hearing Research, 31 (2), 240-252.
Willemsen-Swinkels, S., Buitelaar, J., Weijnen, F., & van Engeland, H. (1998). Timing of social gaze behavior in children with a pervasive developmental disorder. Journal of Autism and Developmental Disorder, 28 (3), 199-210.
Williams, S., Sanderson, G., & Share, D. (1988). Refractive error, IQ and reading ability: A longitudinal study from age seven to 11. Developmental Medicine & Child Neurology, 30 (6), 735-742.
Windsor, J., Doyle, S., Siegel, G. (1994). Language acquisition after mutism: A longitudinal case study of autism. Journal of Speech & Hearing Research, 37 (1), 96-105.
Young, F. (1963). Reading, measures of intelligence and refractive errors. American Journal of Optometry & Archives of the American Academy of Optometry, 40 (4), 257-264.
Zinober, B., & Martlew, M. (1985). Developmental changes in four types of gesture in relation to acts and vocalizations from 10 to 21 months. British Journal of Developmental Psychology, 3 (3), 293-306.
APPENDIX A Consent Form for Children with Disabilities
Consent form for your child to be in a research study:
“The Role of Vision in Language Learning”
(for parents of children with special needs)
~~~~~~~~~~~~~~~~~~~~~Sign and Return THIS page.
You may keep the next page for your records.
~~~~~~~~~~~~~~~~~~~~~~~~~~Statement of Consent:
I have read the information on this study and asked any questions that I may have and received answers. I realize that I or my child may withdraw consent at any time, and that withdrawal from the study does not affect my relationship with the University of Minnesota or my child’s school district. I give my consent for __________ (child’s name) to participate in the study.
Signature of parent or guardian _________ Date ________
Relationship to the child ___________
Signature of Investigator _________________ Date ________
(Martha Low)
I also give my consent for the child’s vision screening results to be placed in the child’s educational file.
Signature of parent or guardian __________ Date ________
Signature of Investigator ______ Date _______
(Martha Low)
You are invited to participate in a research study on the role of vision in language learning. Your child was selected as a possible participant because s/he is enrolled in Early Childhood Special Education. This study is being conducted by Martha Low, doctoral candidate in Educational Psychology, Special Education Programs, at the University of Minnesota.
Background Information: The purpose of this study is to discover how young children use their vision to learn new words. When typical children are 1 ½ years old, they figure out the meaning of new words by looking at the speaker and/or where the speaker is looking. This study seeks to understand how children with special needs differ from typical children in the use of this visual strategy. In addition, poor eyesight (e.g., near-sightedness or far-sightedness) could make it more difficult for children to follow a speaker’s gaze. Children with special needs are more likely to have vision problems. The study will try to determine if there is a relationship between vision problems and language learning in children with special needs.
Procedures: If you agree to let your child participate in this study, we would ask your consent for the following: (1) The researcher will play with your child using unfamiliar toys which will be given made-up names. After playing, the child will be asked to choose an unfamiliar toy from a group of toys when the researcher uses its made-up name. (2) Your child will be photographed with a vision screening camera (5 minutes). (3) If your child is unable to find the familiar toy, the child will play the language game again on another day (additional 15 minutes). The procedures will be changed slightly to help the child associate the unfamiliar toy with its made-up name (e.g., use a pointing gesture). A videotape will be made of the play times. Information from the videotapes will be compared with typical children. The researcher will coordinate with the child’s teacher in order to ensure that the child does not miss teaching opportunities for his/her educational goals. In the event that the child becomes unhappy (e.g., bored, irritated) with the play time or the vision screening, attempts will be made to make the task more enjoyable. The task will be stopped if the child continues to protest or is unwilling to participate.
Risks of Being in the Study: Your child may find the bright flash of the vision screening camera uncomfortable. The vision screening camera flash is similar to an ordinary camera flash. There is no physical risk to the child.
Benefits of Being in the Study: Results from the vision test may indicate that the child has a problem seeing. You will be notified of the child’s vision test results in writing. If you use this information to have your child seen by an optometrist or a medical professional, vision problems might be corrected.
Confidentiality: Your signature permits the researcher (Martha Low) access to information from your child’s educational records. This will make certain that your child meets the criteria for the study and can perform the language tasks. Only information which is pertinent to the study will be recorded from the files. If you agree (second signature below), a copy of the vision test results and the photograph will be placed in your child’s educational file. In addition, each child in the study will receive a number which will be used instead of his/her name. These numbers will be used on written records, videotapes, and vision screening photos. Information about the study will be reported in a way which will not permit the identification of any individual child.
Voluntary Nature of the Study: Your decision whether or not to allow your child to participate will not affect your current or future relations with your child’s school or the University of Minnesota. You are free to withdraw your approval at any time without affecting those relationships. Withdrawal from the study will not prevent vision test results from being shared with the school if you have given your consent (second signature below). The child can also end participation through indications of irritability or unhappiness.
Contacts and Questions: Feel free to contact the following individuals with any questions.
Martha Low (researcher) Susan Hupp, Ph.D. (advisor)
Phone: XXXXXXXXXXX (office) Phone:XXXXXXXXXX (office)
Phone: XXXXXXX (home) Email:Email:XXXXXXXXXX
Mail: University of Minnesota, Department of Educational Psychology/Special Education
Programs, College of Education and Human Development, Burton 227, 178 Pillsbury
Drive, S.E., Minneapolis, MN, 55455 (Attn: Martha Low)
If you have any questions or concerns regarding this study and would like to talk to someone other than the researchers, contact Research Subjects’ Advocate line, D528 Mayo, 420 Delaware Street Southeast, Minneapolis, Minnesota 55455; telephone (612) 625-1650.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Statement of Consent – DO NOT SIGN OR RETURN THIS PAGE – (this is for your records):
I have read the information on this study and asked any questions that I may have and received answers. I realize that I or my child may withdraw consent at any time, and that withdrawal from the study does not affect my relationship with the University of Minnesota or my child’s school district. I give my consent for _______________ (child’s name) to participate in the study.
Signature of parent or guardian _______________________________Date ________________
Relationship to the child _________
Signature of Investigator __________________________Date ________________
(Martha Low)
I also give my consent for the child’s vision screening results to be placed in the child’s educational file.
Signature of parent or guardian _______________________________Date ________________
Signature of Investigator ____________________________________Date ________________
(Martha Low)
APPENDIX B - Consent Form for Typical Children
CONSENT FORM FOR RESEARCH STUDY ON THE ROLE OF VISION IN LANGUAGE IN LEARNING(for parents of children without disabilities)
You are invited to participate in a research study on the role of vision in language learning. Your child was selected as a possible participant because s/he comes to a church nursery program. We ask that you read this form and ask any questions you may have before agreeing to be in the study. This study is being conducted by: Martha Low, doctoral candidate in Educational Psychology, Special Education Programs, at the University of Minnesota.
Background Information:
The purpose of this study is to discover how young children use their vision to learn new words. When typical children are 1 ½ years old, they figure out the meaning of new words by looking at the speaker and/or where the speaker is looking. This study seeks to understand how children with moderate to severe disabilities differ from typical children in the use of this visual strategy. In addition, poor eyesight (e.g., near-sightedness or far-sightedness) could make it more difficult for children to follow a speaker’s gaze, so participants will be given a vision test using a special camera. Children with moderate to severe disabilities are more likely to have vision problems, but occasionally typical children have them too. The study will try to determine if there is a relationship between vision problems and language learning in children with moderate to severe disabilities.
Procedures:
If you agree to let your child participate in this study, we would ask your consent for the following: (1) The researcher will play with your child for about 15 minutes. During that time, familiar and unfamiliar toys will be used. After the child has heard the researcher give unfamiliar names for the unfamiliar toys, the child will be asked to play a finding game, where the child is asked to identify the unfamiliar toys. (2) Your child will be photographed with a vision screening camera. This camera works the like a regular camera, but it only takes pictures of the eyes. This will take about 5 minutes, which allows time for the children to enjoy watching the photos develop. (3) If your child is unable to find the familiar toy, the child will play the language game again on another day. The procedures will be changed slightly to help the child associate the new toy with the new word (e.g., add a pointing gesture, repeat the new word more often). This would take another 15 minutes. A videotape will be made of all play times. Information from the videotapes will be compared with typical children.
The researcher will coordinate with the child’s teacher in order to ensure that the child does not miss teaching opportunities for his/her educational goals. In the event that the child becomes unhappy (e.g., bored, irritated) with the play time or the vision screening, two attempts will be made to make the task more enjoyable. The task will be stopped if the child continues to protest or is unwilling to participate. One attempt will be made to retest the child at another time. If the child still expresses displeasure, study participation will be discontinued.
Risks of Being in the Study:
The study has a small risk; your child may find the flash of the vision screening camera uncomfortable. The vision screening camera flash is similar to an ordinary camera flash, and there are two of these flashes for each vision screening photograph. Occasionally, a vision screening must be repeated. No more than two pictures will be taken of a child on one day. There is no physical risk to the child. Retakes are estimated to be about 1 in 50. Martha Low is certified by the Department of Health to conduct vision screenings and interpret the photographs.
Benefits of Being in the Study:
Screening results may indicate that the child has a visual impairment (e.g., cataracts or strabismus) or a problem with visual acuity (e.g., near-sighted, far-sighted, astigmatism). Children who have problems with acuity can be further tested by an optometrist and fitted for glasses. Children with more serious eye conditions such as cataracts or strabismus can be referred for medical treatment. You will be notified of the child’s vision screening results in writing.
Confidentiality:
Each child in the study will receive a number which will be used instead of his/her name. These numbers will be used on written records, videotapes, and vision screening photos. The sheet of paper which matches the children’s names and numbers will be kept separate from the data in the researcher’s locked file cabinet. Information about the study will be reported in a way which will not permit the identification of any individual child. All records, data files, photos, and videotapes from the study will be kept indefinitely in the researcher’s locked office.
Voluntary Nature of the Study:
Your decision whether or not to participate will not affect your current or future relations with your child’s church nursery program or the University of Minnesota. If you decide to participate, you are free to withdraw at any time without affecting those relationships. Withdrawal from the study will not result in a loss of benefits for the child. The child also may choose to end participation in the study through nonverbal indications of unwillingness to comply. Any verbal or written communication from parents will also be honored.
Contacts and Questions: Feel free to contact the following individuals with any questions.
Martha Low (researcher) Susan Hupp, Ph.D. (advisor)
Phone: XXXXXXXX (office) Phone: XXXXXXXX (office)
Phone: XXXXXXX (home) Email: XXXXXXXX
Email: XXXXXXXXX Mail: same (attn: Susan Hupp)
Mail: University of Minnesota
Department of Educational Psychology/Special Education Programs
College of Education and Human Development
Burton 227
178 Pillsbury Drive, S.E.
Minneapolis, MN, 55455
(Attn: Martha Low)
If you have any questions or concerns regarding this study and would like to talk to someone other than the researchers, contact Research Subjects’ Advocate line, D528 Mayo, 420 Delaware Street Southeast, Minneapolis , Minnesota 55455; telephone (612) 625-1650.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Sign and Return the NEXT page.
(The last page is a copy of this page.)
You may keep this form for your records.
~~~~~~~~~~~~~~~~~~~~~
Statement of Consent:
I have read the above information. I have asked any questions that I may have and received answers. I give my consent for __________________________ (child’s name) to participate in the study.
Signature of parent or guardian _________________________ Date ____________
Relationship to the child _______________________________
Signature of Investigator _______________________________ Date ________________
(Martha Low)
English is the language spoken at the child’s home (please check one).
__________ YES ____________ NO
If you attend Bloomington Assembly of God Church on Sunday, please check the times you are in attendance (for alternate testing days).
____ 1st service _____School of the Bible Hour ____ 2nd Service
~~~~~~~~~~~~~~~~
Sign and Return this page ONLY.
You may keep this form (the other pages) for your records.
~~~~~~~~~~~~~~~~
Statement of Consent:
I have read the above information. I have asked any questions that I may have and received answers. I give my consent for _______________ (child’s name) to participate in the study.
Signature of parent or guardian _______________________________ Date ________
Relationship to the child _____________________________________
Signature of Investigator ____________________________________ Date ________________
(Martha Low)
English is the language spoken at the child’s home (please check one).
__________ YES ____________ NO
If you attend Bloomington Assembly of God Church on Sunday, please check the times you are in attendance (for alternate testing days).
____ 1st service _____School of the Bible Hour ____ 2nd Service
APPENDIX C -Data Collection Sheets
Number:
Date of File Info:
Date of Birth: Test Date: Total Age in months:
Child’s sex? M F English spoken in the home? yes no
Disability (sped service): Diagnosis:
Mental Age: Developmental Level: IQ score:
Total Language Score: Expressive: Receptive:
Visual Info: Prescribed Glasses? yes no Wears? seatwork
If yes, for distance near-vision up&around
_____ hrs.
Auditory Info: Voice Range Loss? Amplification? yes no
If yes, with peers talking
teacher talking
If yes, time used?
____hrs.
Motor Info: Gross? Fine?
[Photorefraction] date(s) of testing ________________________________
+ -- ______ (#) on the Myopia-Hyperopia continuum
Group: Myopic Normal Hyperopic
(-.25 & more) (+ 2.0 & more)
Other visual information revealed in photos:
Pupil Alignment?
Pupil Size?
Pupils Equal in Size?
Pupil Opacities & Iris Irregularities?
Astigmatism?
Amblyopia?
Strabismus?
Near-Sighted?
Far-Sighted?
Normal?
[Warm-up] Counterbalancing string #: 1 2 3 4 5 6
1st, Pair __, ______, on my R L correct? Y N B 0 gesture? Y N vocal? Y N child used R L B 0
_______, on my R L correct? Y N B 0 gesture? Y N vocal? Y N child used R L B 0
2nd, Pair __, ______, on my R L correct? Y N B 0 gesture? Y N vocal? Y N child used R L B 0
_______, on my R L correct? Y N B 0 gesture? Y N vocal? Y N child used R L B 0
3rd, Pair __, ______, on my R L correct? Y N B 0 gesture? Y N vocal? Y N child used R L B 0
_______, on my R L correct? Y N B 0 gesture? Y N vocal? Y N child used R L B 0
How many items until the game was learned? 0 1st 2nd 3rd 4th 5th 6th
Word(s) successfully responded to? car - shoe baby - boat ball - phone
How was object indicated? pick-up touch show give indicate point
How many trials were successful? 0 1 2 3 4 5 6
Percentage (%) gesture used (of successful trials)?
Percentage (%) vocalization used (of successful trials)?
Position bias demonstrated? R L No
Child used R L B 0
[Phase 1] date(s) of testing ________________________________
Counterbalancing string #: 1 2 3 4 5 6 7 8
· A: Follow-in: 1st 2nd word: Peri Toma novel toy: 1 2 3 4
/5 looks to researcher
/5 looks to researcher’s toy only
/5 looks sequentially to researcher and toy of focus
/5 inaccurate gaze following
/5 no response
/5 protocol breach
Right-Left Orientation – focal toy is on researcher’s Right Left
“[Name], where’s the [word]?”
(1) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“[Name], look at the [word]!”
(1) looks to the R L B 0 I Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) looks to the R L B 0 I Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“[Name], where’s the [word]?”
(1) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“Is this the [word]?” (to the correct/incorrect object) affirms disaffirms B 0
or (2) Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0 Head Nod? Y N
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
· Test Fast-Map Association (“Gonna find it”) for A: Follow-in
(1) Presented on my R L correct? Y N B 0 E Vocalize? Y N Gesture? Y N used R L B N
(2) Presented on my R L correct? Y N B 0 E Vocalize? Y N Gesture? Y N used R L B N
How many trials were successful? 2right 1right B/0 1wrong 2wrongtoy
Position bias demonstrated? R L No
Child used R L B 0
How was object indicated? pick-up touch show give indicate point
· B: Discrepant: 1st 2nd word: Peri Toma novel toy: 1 2 3 4
/5 looks to researcher
/5 looks to researcher’s toy only
/5 looks sequentially to researcher and toy of focus
/5 inaccurate gaze following
/5 no response
/5 protocol breach
Right-Left Orientation: focal toy is on the Right Left
“[Name], where’s the [word]?”
(1) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“[Name], look at the [word]!”
(1) looks to the R L B 0 I Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) looks to the R L B 0 I Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“[Name], where’s the [word]?”
(1) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“Is this the [word]?” (to the correct/incorrect object) affirms disaffirms B 0
or (2) Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0 Head Nod? Y N
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
· Test Fast-Map Association (“Gonna find it”) for B: Discrepant
(1) Presented on my R L correct? Y N B 0 E Vocalize? Y N Gesture? Y N used R L B N
(2) Presented on my R L correct? Y N B 0 E Vocalize? Y N Gesture? Y N used R L B N
How many trials were successful? 2right 1right B/0 1wrong 2wrong
Position bias demonstrated? R L No
Child used R L B 0
How was object indicated? pick-up touch show give indicate point
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
· Was child comfortable with the testing?
Follow-in? Yes Anxious Restless 1st condition? Yes Anxious Restless
Discrepant? Yes Anxious Restless 2nd condition? Yes Anxious Restless
· Was child interactive? 1 2 3 4 5
· Was child verbally skilled? 1 2 3 4 5
Number: ______________ Date of testing ____________
[Phase 2] Counterbalancing string #: 1 2 3 4
· C: Add Head Turn: 1st 2nd word: Neffer Boka novel toy: 5 6 7 8
Right-Left Orientation – focal toy is on researcher’s Right Left
· Eye Gaze
[using eye gaze] “[Name], look at the [word]!”
(1) looks to the R L B 0 I Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) looks to the R L B 0 I Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“[Name], where’s the [word]?”
(1) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“Is this the [word]?” (to the correct/incorrect object) affirms disaffirms B 0
Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0 Head Nod? Y N
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
[If they get it right with eye gaze only…..]
· Test Fast-Map Association (“Gonna find it”) for Eye Gaze
(1) Presented on my R L correct? Y N B 0 E Vocalize? Y N Gesture? Y N used R L B N
(2) Presented on my R L correct? Y N B 0 E Vocalize? Y N Gesture? Y N used R L B N
How many trials were successful? 2right 1right B/0 1wrong 2wrong
Position bias demonstrated? R L No
Child used R L B 0
How was object indicated? pick-up touch show give indicate point
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
· Head Turn
[using head turn] “[Name], look at the [word]!”
(1) looks to the R L B 0 I Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) looks to the R L B 0 I Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“[Name], where’s the [word]?”
(1) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“Is this the [word]?” (to the correct/incorrect object) affirms disaffirms B 0
Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0 Head Nod? Y N
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
· Test Fast-Map Association (“Gonna find it”) for Head Turn
(1) Presented on my R L correct? Y N B 0 E Vocalize? Y N Gesture? Y N used R L B N
(2) Presented on my R L correct? Y N B 0 E Vocalize? Y N Gesture? Y N used R L B N
How many trials were successful? 2right 1right B/0 1wrong 2wrong
Position bias demonstrated? R L No
Child used R L B 0
How was object indicated? pick-up touch show give indicate point
· D: Add Gesture: 1st 2nd word: Neffer Boka novel toy: 5 6 7 8
Right-Left Orientation – focal toy is on researcher’s Right Left
· Eye Gaze
[using eye gaze] “[Name], look at the [word]!”
(1) looks to the R L B 0 I Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) looks to the R L B 0 I Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“[Name], where’s the [word]?”
(1) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“Is this the [word]?” (to the correct/incorrect object) affirms disaffirms B 0
Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0 Head Nod? Y N
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
[If they get it right with eye gaze only…..]
· Test Fast-Map Association (“Gonna find it”) for Eye Gaze
(1) Presented on my R L correct? Y N B 0 E Vocalize? Y N Gesture? Y N used R L B N
(2) Presented on my R L correct? Y N B 0 E Vocalize? Y N Gesture? Y N used R L B N
How many trials were successful? 2right 1right B/0 1wrong 2wrong
Position bias demonstrated? R L No
Child used R L B 0
How was object indicated? pick-up touch show give indicate point
~~~~~~~~~~~~~~~~~~~~~~~~
· Show Gesture
[using gesture] “[Name], look at the [word]!”
(1) looks to the R L B 0 I Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) looks to the R L B 0 I Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“[Name], where’s the [word]?”
(1) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
(2) chooses R L B 0 E Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0
“Is this the [word]?” (to the correct/incorrect object) affirms disaffirms B 0
Correct? Y N B 0 Vocalize? Y N Gesture? Y N used R L B 0 Head Nod? Y N
~~~~~~~~~~~~~~~~~~~~~~~
· Test Fast-Map Association (“Gonna find it”) for C: Gesture
(1) Presented on my R L correct? Y N B 0 E Vocalize? Y N Gesture? Y N used R L B N
(2) Presented on my R L correct? Y N B 0 E Vocalize? Y N Gesture? Y N used R L B N
How many trials were successful? 2right 1right B/0 1wrong 2wrong
Position bias demonstrated?R L No
Child used R L B 0
~~~~~~~~~~~~~~~~~~~~~~~~
· Was child comfortable with the testing?
Head turn? Yes Anxious Restless
Gesture? Yes Anxious Restless
1st condition? Yes Anxious Restless
2nd condition? Yes Anxious Restless
· Was child interactive? 1 2 3 4 5
· Was child verbally skilled? 1 2 3 4 5
~~~~~~~~~~~~~~~~~~~~~~~~
Key for coding videotaped data:
Y = the right object chosen/looked at R = object positioned on researcher’s right
N = the wrong object chosen/looked at or, child looks to researcher’s right
B = both objects chosen/looked at L = object positioned on researcher’s left
0 = neither object chosen/looked at or, child looks to researcher’s left
I = child looks in correct direction E = child chooses an environmental location
but overshoots the target object matching a previous looking trial where
the child overshot the target object
· Was child interactive? 1 2 3 4 5
1 = hardly responsive to my prompts
2 = sometimes responsive to my prompts
3 = responsive to my prompts
4 = responsive and initiates some interaction
5 = responsive and initiates much interaction
· Was child verbally skilled? 1 2 3 4 5
1 = no vocalization
2 = some vocalization
3 = often vocalizes
4 = uses some words
5 = often uses words
APPENDIX D - Procedural Flow Charts
APPENDIX E
Procedural Flow Chart for Warm-up Activity and Phase 1
The Warm-Up Strings (WU)
WU 1 - IR, 2L, 3R
WU 2 - 1L, 3R, 2L
WU 3 - 2R, 3L, 1R
WU 4 - 2L, 1R, 3L
WU 5 - 3R, 1L, 2R
WU 6 - 3L, 2R, 1L
Key: 1-3 = pair numbers.
L = ask for left position toy first.
R = ask for right position toy first.
The Phase 1 Strings (P1)
P 1/1 - F.1.PERI.R; D.E. Toma
P 1/2 - D/2.Toma.R; F.4.Peri.L
P 1/3 - F.1.Toma.L; D.E.Peri.R
P 1/4 - D.2.Peri.L;F.4.Toma.R
P 1/5 -F.2.Peri.R; D.4.Peri.R.
P 1/6 -D.1.Toma.R;F.3.Peri.L
P 1/7 - F.2.Toma.L;D.4.Peri.R
P 1/8 - D.1.Peri.L; F.3.Toma.L
Key: F=follow-in condition.
D=discrepant condition.
1-4=novel toys.
Toma & Peri=novel labels.
R=move toy to right position.
L=move toy to left position.
(The cue card depicts an ariel view of the researcher and child at the card table.)
Procedural Flow Chart for Warm-up Activity and Phase 2
The Phase 2 Strings (P2):
P2/1 - H.5.Boka.R; G.7.Neffer.L
P2/2 - G.6.Neffer.R; H.8.Boka.L
P2/3 - H.5.Neffer.L;G.7.Boka.R
P2/4 - G.6.Boka.L;H.8.Neffer.R
H = head turn condition.
G = show gesture condition.
5-8 = novel toys.
Neffer & Boka = novel labels.
R = move toy to right position.
L = move toy to left position.
APPENDIX F
CLICK HERE FOR PRINTER-FRIENDLY VERSION.
GLOBALIZATION AND CULTURAL TERRITORIES | THE ROLE OF VISION IN LANGUAGE LEARNING
- in Children with Moderate to Severe Disabilities | EARLY GANDHI AND THE LANGUAGE POLICY
OF THE INDIAN NATIONAL CONGRESS | AN ASSESSMENT OF FACTORS ASSOCIATED WITH STUDENTS POOR PERFORMANCE - IN SENIOR SCHOOL CERTIFICATE ENGLISH LANGUAGE IN NIGERIA ... | ENGLISH PUN AND ITS CLASSIFICATION |A COMPREHENSIVE
STUDY ON THE FORMATION OF COMPOUND VERBS IN TAMIL |LANGUAGE NEWS THIS MONTH - SINDH IN THE NATIONAL ANTHEM, FEDERAL POLITY, LINGUISTIC ISLANDS, ETC. |HOME PAGE | CONTACT EDITOR
Martha Low, Ph.D.
C/o. Language in India
Send your articles
as an attachment
to your e-mail to
thirumalai@bethfel.org.

